
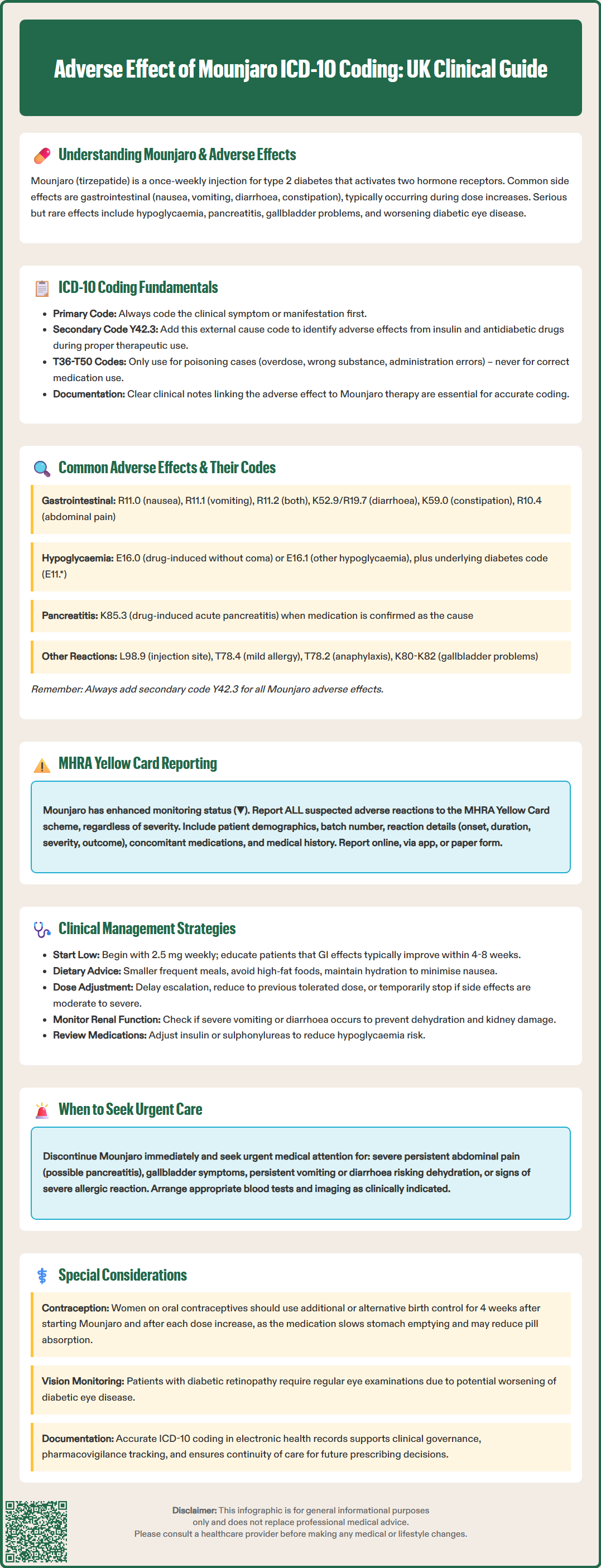
Mounjaro (tirzepatide) is a dual GIP and GLP-1 receptor agonist licensed in the UK for type 2 diabetes mellitus management. Accurate documentation of adverse effects using ICD-10 coding is essential for clinical governance, pharmacovigilance, and patient safety. When documenting adverse effects of Mounjaro in UK practice, clinicians must code the clinical manifestation first, followed by the external cause code Y42.3 (Insulin and oral hypoglycaemic drugs causing adverse effects in therapeutic use). This article provides comprehensive guidance on recognising, coding, and managing Mounjaro-related adverse reactions in accordance with NHS coding standards and MHRA reporting requirements.
Quick Answer: The ICD-10 code for adverse effects of Mounjaro (tirzepatide) in UK practice is Y42.3, used as a secondary code after the manifestation code describing the specific clinical symptom.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereMounjaro (tirzepatide) is a novel glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist licensed in the UK for the treatment of type 2 diabetes mellitus in adults. Administered as a once-weekly subcutaneous injection, tirzepatide works through dual incretin receptor activation, enhancing insulin secretion in a glucose-dependent manner whilst suppressing glucagon release and delaying gastric emptying. This mechanism contributes to improved glycaemic control and weight reduction, as demonstrated in clinical trials.
As with all medications, Mounjaro carries a risk of adverse effects that clinicians must recognise and manage appropriately. The most frequently reported adverse reactions are gastrointestinal in nature, including nausea, vomiting, diarrhoea, constipation, and abdominal discomfort. These effects typically emerge during dose escalation and often diminish over time as patients develop tolerance. However, some individuals may experience persistent or severe symptoms requiring dose adjustment or discontinuation.
Less common but clinically significant adverse effects include hypoglycaemia (particularly when used alongside insulin or sulphonylureas), acute pancreatitis, gallbladder disorders, and hypersensitivity reactions. There have also been reports of diabetic retinopathy complications in patients with pre-existing retinal disease, necessitating appropriate monitoring and prompt reporting of visual changes.
Importantly, the delayed gastric emptying effect of tirzepatide may reduce the absorption of oral medications, including oral contraceptives. Women using oral contraceptives should be advised to consider non-oral or additional barrier contraception for 4 weeks after initiation and following each dose increase. Severe gastrointestinal symptoms may lead to dehydration and acute kidney injury; patients should be counselled to maintain adequate hydration and seek medical advice if persistent vomiting or diarrhoea occurs.
The International Classification of Diseases, 10th Revision (ICD-10) provides a standardised framework for documenting adverse drug reactions in clinical records and healthcare databases across the UK. Accurate coding is essential for epidemiological surveillance, clinical audit, reimbursement processes, and pharmacovigilance activities.
In UK clinical coding practice, adverse effects of medications that were correctly prescribed and properly administered follow a specific coding pattern according to the NHS National Clinical Coding Standards. When documenting adverse effects of Mounjaro, clinicians should first code the manifestation (the clinical presentation or symptom) followed by an external cause code from the Y40-Y59 range to indicate that the manifestation was caused by a medication in therapeutic use.
For Mounjaro-related adverse effects, the appropriate external cause code is Y42.3 (Insulin and oral hypoglycaemic [antidiabetic] drugs causing adverse effects in therapeutic use). This code should be assigned as a secondary code after the manifestation code.
It is important to note that in UK coding practice, codes from the T36-T50 range are reserved for poisoning (overdose, wrong substance given or taken in error, or wrong route of administration) and should not be used for adverse effects occurring during correct therapeutic use. This differs from some international coding practices.
Accurate documentation in the clinical notes is essential to support appropriate coding, clearly stating the suspected adverse effect and its relationship to Mounjaro therapy. Clinicians should follow local NHS clinical coding policies, which may provide specific guidance on coding adverse drug reactions.
Gastrointestinal adverse effects represent the most prevalent category of Mounjaro-related reactions. In UK ICD-10 coding, nausea should be coded as R11.0 (Nausea), whilst vomiting is documented as R11.1 (Vomiting). When both symptoms occur concurrently, R11.2 (Nausea with vomiting) provides a combined code. For diarrhoea related to medication, K52.9 (Noninfective gastroenteritis and colitis, unspecified) or R19.7 (Diarrhoea, unspecified) may be appropriate depending on clinical context. Constipation is classified under K59.0. Abdominal pain, another frequent complaint, is coded as R10.4 (Other and unspecified abdominal pain), with more specific subcategories available depending on the location and characteristics of the pain.
Hypoglycaemia associated with Mounjaro, particularly when used in combination with other glucose-lowering agents, should be coded as E16.0 (Drug-induced hypoglycaemia without coma) or E16.1 (Other hypoglycaemia) as appropriate, with additional diabetes codes (E11.) to record the underlying condition. Acute pancreatitis, a serious but uncommon adverse effect, is documented using K85.3* (Drug-induced acute pancreatitis) when medication causality is established.
Injection site reactions are coded according to the specific dermatological manifestation, such as L98.9 (Disorder of the skin and subcutaneous tissue, unspecified) or more specific codes if the presentation is clearly defined. Allergic reactions range from mild hypersensitivity (T78.4, Allergy, unspecified) to severe anaphylaxis (T78.2, Anaphylactic shock, unspecified). Gallbladder-related complications, including cholelithiasis and cholecystitis, are coded within the K80-K82 range.
For each adverse effect, the manifestation code should be followed by Y42.3 (Insulin and oral hypoglycaemic [antidiabetic] drugs causing adverse effects in therapeutic use) to establish the causal relationship with Mounjaro therapy.
Healthcare professionals and patients in the UK have a professional and civic responsibility to report suspected adverse drug reactions to the Medicines and Healthcare products Regulatory Agency (MHRA) through the Yellow Card scheme. This pharmacovigilance system is crucial for identifying previously unrecognised adverse effects, monitoring the safety profile of newly licensed medications like Mounjaro, and detecting rare but serious reactions that may not have been apparent during clinical trials.
For Mounjaro, which received UK marketing authorisation relatively recently, the MHRA encourages reporting of all suspected adverse reactions, regardless of severity. This enhanced monitoring is indicated by a black triangle (▼) symbol in the product literature, though clinicians should check the current SmPC to confirm the monitoring status. Reports can be submitted online via the Yellow Card website (https://yellowcard.mhra.gov.uk), through the Yellow Card app, or by completing a paper form. When submitting a report, clinicians should provide comprehensive information including patient demographics (age, sex), the suspected medication (including batch number if available), details of the adverse reaction (onset, duration, severity, outcome), concomitant medications, and relevant medical history.
Accurate ICD-10 coding in electronic health records facilitates systematic identification of adverse drug reactions for both local clinical governance and national pharmacovigilance purposes. Clinicians should document adverse effects promptly and thoroughly in patient records, ensuring that both the clinical manifestation and the suspected causative agent are clearly recorded. This documentation supports continuity of care, informs future prescribing decisions, and contributes to the broader understanding of Mounjaro's safety profile in real-world clinical practice.
Effective management of Mounjaro adverse effects begins with comprehensive patient education prior to treatment initiation. Patients should be counselled about common gastrointestinal symptoms, advised to start with the lowest dose (2.5 mg weekly), and informed that side effects typically improve over 4-8 weeks as tolerance develops. Practical strategies to minimise nausea include eating smaller, more frequent meals, avoiding high-fat foods, and ensuring adequate hydration. If gastrointestinal symptoms are troublesome but tolerable, maintaining the current dose for an additional week or two before escalation may improve tolerance.
When adverse effects are moderate to severe, dose modification represents the first-line management strategy. Clinicians may choose to delay dose escalation, reduce to the previous tolerated dose, or temporarily discontinue treatment to allow symptom resolution. According to NICE guidance on diabetes management, treatment decisions should be individualised, balancing glycaemic control benefits against tolerability concerns. For persistent nausea, short-term antiemetic therapy may be considered, though with important restrictions: metoclopramide should be limited to a maximum 5-day course with appropriate dose limits as per MHRA guidance, whilst domperidone should only be used when benefits clearly outweigh risks, with assessment for cardiac risk factors and QT prolongation.
Serious adverse effects require prompt intervention. Patients experiencing severe, persistent abdominal pain (potentially indicating pancreatitis) should discontinue Mounjaro immediately and seek urgent medical assessment, including serum amylase or lipase measurement. Similarly, symptoms suggestive of gallbladder disease warrant investigation with liver function tests and abdominal ultrasonography. Hypoglycaemia management follows standard protocols, with review of concomitant glucose-lowering medications and potential dose reduction of insulin or sulphonylureas.
Patients should be advised to maintain adequate hydration, particularly if experiencing persistent vomiting or diarrhoea, and clinicians should consider monitoring renal function if severe gastrointestinal symptoms occur. Women of childbearing potential should be counselled about potential reduced efficacy of oral contraceptives; barrier or non-oral contraceptive methods are recommended for 4 weeks after initiation and following each dose increase. Regular monitoring, including review of symptoms, weight, HbA1c, and appropriate screening for diabetic retinopathy in those with pre-existing disease, enables early identification and management of adverse effects whilst optimising therapeutic outcomes.
In UK practice, code the clinical manifestation first (e.g., R11.0 for nausea), followed by Y42.3 (Insulin and oral hypoglycaemic drugs causing adverse effects in therapeutic use) as the secondary external cause code.
No, T36-T50 codes are reserved for poisoning (overdose, wrong substance, or wrong route) in UK coding practice. For adverse effects during correct therapeutic use, code the manifestation followed by Y42.3.
The most frequent adverse effects are gastrointestinal: nausea (R11.0), vomiting (R11.1), diarrhoea (K52.9/R19.7), and constipation (K59.0). All should be followed by Y42.3 to indicate medication causality.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.