LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

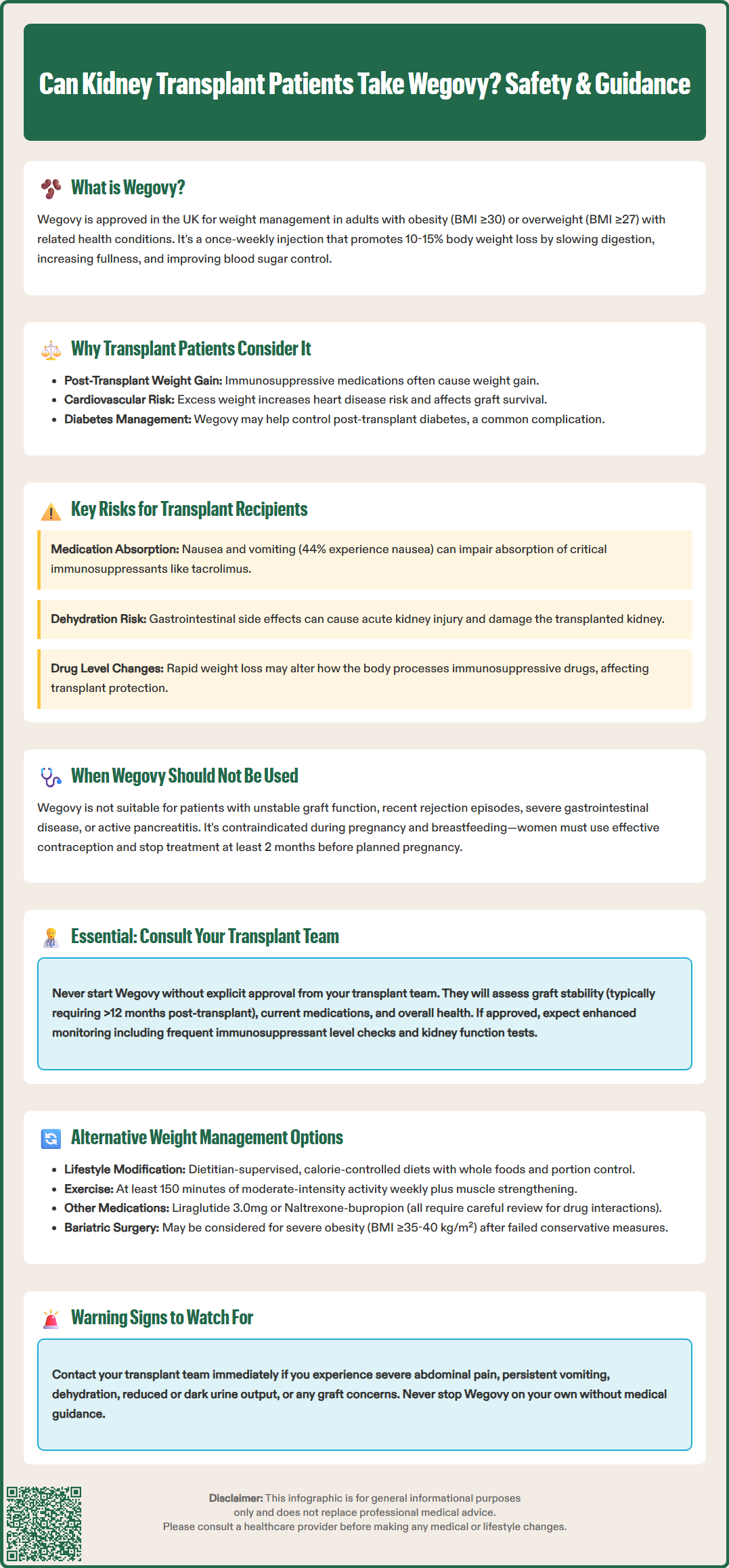
Wegovy (semaglutide 2.4 mg) is a glucagon-like peptide-1 (GLP-1) receptor agonist licensed in the UK for weight management in adults with obesity or overweight with weight-related comorbidities. For kidney transplant recipients, the decision to use Wegovy requires careful consideration and must be made in consultation with the transplant team, as there is limited clinical data specifically evaluating its safety and efficacy in this population. Obesity after transplantation can increase cardiovascular risk and worsen metabolic complications, making weight management clinically important. However, the use of newer anti-obesity medications like Wegovy has not been extensively studied in transplant recipients, necessitating an individualised, cautious approach with enhanced monitoring.
Quick Answer: Kidney transplant patients may use Wegovy, but only with explicit approval from their transplant team, as there is limited clinical data in this population and careful monitoring of graft function and immunosuppressant levels is essential.
Wegovy (semaglutide 2.4 mg) is a glucagon-like peptide-1 (GLP-1) receptor agonist licensed in the UK for weight management in adults with obesity (BMI ≥30 kg/m²) or overweight (BMI ≥27 kg/m²) with weight-related comorbidities, as an adjunct to reduced-calorie diet and increased physical activity. For kidney transplant recipients, the decision to use Wegovy requires careful consideration and must be made in consultation with the transplant team, as there is limited clinical data specifically evaluating its safety and efficacy in this population.
Kidney transplant patients represent a unique clinical group who often experience weight gain following transplantation, partly due to immunosuppressive medications such as corticosteroids and improved overall health. Obesity after transplantation can increase cardiovascular risk, worsen metabolic complications including post-transplant diabetes mellitus, and potentially affect long-term graft survival. Whilst weight management is clinically important in this population, the use of newer anti-obesity medications like Wegovy has not been extensively studied in transplant recipients.
Current evidence and regulatory position:
There is very limited data on semaglutide use in solid organ transplant recipients
There is no official contraindication to use in transplant patients in the UK Summary of Product Characteristics
Individual case reports and small observational studies are beginning to emerge
NICE guidance (TA875) specifies that semaglutide for weight management should be initiated within specialist weight management services and is usually limited to 2 years of treatment
The decision to prescribe Wegovy to a kidney transplant patient should be individualised, taking into account the patient's overall clinical status, time since transplantation, stability of graft function, concurrent medications, and cardiovascular risk profile. Close monitoring by the transplant team is essential if treatment is initiated.
Importantly, Wegovy is contraindicated during pregnancy and should be discontinued at least 2 months before a planned pregnancy. Women of childbearing potential should use effective contraception while taking Wegovy. The medication should not be used during breastfeeding.
Wegovy contains semaglutide, a GLP-1 receptor agonist that mimics the action of the naturally occurring incretin hormone GLP-1. The medication works through several mechanisms to promote weight loss: it slows gastric emptying, increases satiety, reduces appetite through central nervous system effects, and improves glycaemic control by enhancing glucose-dependent insulin secretion whilst suppressing inappropriate glucagon release.
Administered once weekly via subcutaneous injection, Wegovy follows a specific dose escalation schedule: 0.25 mg for weeks 1-4, 0.5 mg for weeks 5-8, 1.0 mg for weeks 9-12, 1.7 mg for weeks 13-16, and the maintenance dose of 2.4 mg from week 17 onwards. If patients do not tolerate a dose increase, it may be delayed by 4 weeks. This gradual escalation helps minimise gastrointestinal side effects. In the general population, the STEP clinical trials demonstrated average weight loss of 10–15% of body weight over 68 weeks, alongside improvements in cardiovascular risk factors including blood pressure, lipid profiles, and glycaemic control.
Potential effects in transplant recipients:
For kidney transplant patients, the metabolic benefits of GLP-1 receptor agonists may be particularly relevant. Many transplant recipients develop post-transplant diabetes mellitus or experience worsening of pre-existing diabetes, conditions for which GLP-1 agonists have established efficacy. The cardiovascular benefits observed in non-transplant populations could theoretically extend to transplant recipients, who face elevated cardiovascular mortality risk.
However, the altered physiology in transplant patients—including changes in drug metabolism, potential drug-drug interactions with immunosuppressants, and the presence of a transplanted organ—means that responses may differ from the general population. While the gastric emptying effects of Wegovy are generally not clinically relevant for most medicines, the gastrointestinal effects, particularly nausea and vomiting, could potentially affect absorption of oral immunosuppressive medications with narrow therapeutic indices such as tacrolimus. Additionally, rapid weight loss might theoretically alter the volume of distribution or metabolism of immunosuppressive drugs, potentially affecting drug levels and graft protection.
Several important safety considerations must be evaluated before initiating Wegovy in kidney transplant recipients. Whilst there is no official contraindication, the lack of robust clinical data in this population necessitates a cautious, individualised approach with enhanced monitoring.
Gastrointestinal adverse effects are very common with Wegovy. According to the Summary of Product Characteristics, these include nausea (reported in approximately 44% of patients), diarrhoea (30%), vomiting (24%), and constipation (24%). For transplant patients taking oral immunosuppressants such as tacrolimus, ciclosporin, mycophenolate, or azathioprine, severe gastrointestinal symptoms could impair medication absorption, potentially leading to subtherapeutic immunosuppression levels and increased rejection risk. Patients experiencing persistent vomiting or diarrhoea should contact their transplant team promptly for assessment and possible immunosuppressant level monitoring.
Drug interactions require careful consideration. Wegovy delays gastric emptying, which may affect the absorption kinetics of oral medications, particularly those with narrow therapeutic indices. Therapeutic drug monitoring of immunosuppressant levels is advisable when initiating or titrating Wegovy, particularly during the first few months of treatment and during episodes of significant gastrointestinal illness.
Renal function considerations are paramount. Wegovy is not contraindicated in chronic kidney disease, and the transplanted kidney typically functions well in stable recipients. However, dehydration secondary to gastrointestinal side effects could precipitate acute kidney injury and compromise graft function. Patients should be counselled on maintaining adequate hydration and recognising warning signs of dehydration, including reduced urine output, dark urine, or dizziness.
Additional risks include:
Pancreatitis (rare but serious adverse effect; avoid in active pancreatitis, use caution with history of pancreatitis)
Gallbladder disease, including cholelithiasis (use caution with history of gallbladder disease)
Hypoglycaemia, particularly in patients taking insulin or sulfonylureas
Potential exacerbation of diabetic retinopathy in patients with pre-existing disease
Wegovy is not recommended in patients with severe gastrointestinal disease, including severe gastroparesis. Those with unstable graft function, recent rejection episodes, or inadequate immunosuppression should not be considered for treatment until their transplant status is optimised.
Wegovy is contraindicated during pregnancy and breastfeeding. Women of childbearing potential should use effective contraception during treatment and discontinue Wegovy at least 2 months before a planned pregnancy.
Patients should be advised to report suspected side effects via the MHRA Yellow Card Scheme (yellowcard.mhra.gov.uk).
For kidney transplant recipients who are not suitable candidates for Wegovy or who prefer alternative approaches, several evidence-based weight management strategies can be effective. A comprehensive, multidisciplinary approach typically yields the best outcomes.
Lifestyle modification remains the cornerstone of weight management after transplantation. Structured dietary interventions, ideally supervised by a dietitian experienced in transplant care, can address the specific nutritional challenges faced by transplant recipients. A balanced, calorie-controlled diet emphasising whole grains, lean proteins, fruits, vegetables, and healthy fats whilst limiting processed foods, added sugars, and excessive sodium is recommended. Portion control and mindful eating strategies can help counteract the increased appetite often associated with corticosteroid therapy.
Physical activity programmes tailored to individual capabilities are essential. The UK Chief Medical Officers and NHS recommend at least 150 minutes of moderate-intensity aerobic activity weekly, alongside muscle-strengthening activities twice weekly. Transplant recipients should gradually increase activity levels under medical supervision, particularly in the early post-transplant period. Cardiac rehabilitation-style programmes may benefit those with cardiovascular comorbidities.
Pharmacological alternatives to Wegovy include:
Orlistat: A lipase inhibitor that reduces dietary fat absorption, licensed for weight management in the UK. It is contraindicated in patients taking ciclosporin and requires careful review of other potential drug interactions. Gastrointestinal side effects remain common.
Liraglutide 3.0 mg (Saxenda): Another GLP-1 receptor agonist with similar considerations to Wegovy but requiring daily rather than weekly injection.
Naltrexone-bupropion (Mysimba): Licensed in the UK for weight management but not currently recommended by NICE for routine NHS commissioning. It has limited data in transplant populations and requires careful consideration of potential interactions and contraindications, including uncontrolled hypertension.
Behavioural interventions, including cognitive behavioural therapy for weight management, can address psychological factors contributing to weight gain. Many transplant recipients benefit from peer support groups or structured weight management programmes.
Bariatric surgery may be considered for transplant recipients with severe obesity (BMI ≥40 kg/m² or ≥35 kg/m² with comorbidities) who have not achieved adequate weight loss through conservative measures. Sleeve gastrectomy is often considered in transplant patients due to fewer concerns about malabsorption affecting immunosuppressant absorption compared to gastric bypass, though this requires specialist multidisciplinary assessment involving both bariatric and transplant teams.
Referral to NHS Tier 3/4 specialist weight management services may be appropriate, following NICE guidance (CG189), though local access criteria may apply.
It is essential that kidney transplant recipients do not initiate Wegovy without explicit approval from their transplant team. Self-prescribing or obtaining Wegovy through non-specialist routes could compromise graft function and patient safety. The transplant team possesses the specialist knowledge required to assess individual suitability and implement appropriate monitoring protocols.
According to NICE guidance (TA875), semaglutide for weight management should be initiated within specialist weight management services, and treatment is usually limited to 2 years. For transplant recipients, this specialist care must be coordinated with the transplant team.
Key aspects your transplant team will evaluate include:
Time since transplantation: Patients in the early post-transplant period (typically <12 months) with evolving immunosuppression regimens are generally not suitable candidates
Graft function stability: Current and trend of estimated glomerular filtration rate (eGFR), proteinuria, and recent rejection history
Immunosuppression regimen: Current medications, recent dose adjustments, and therapeutic drug level stability
Comorbidity profile: Presence of post-transplant diabetes, cardiovascular disease, or other conditions that might influence risk-benefit assessment
Concurrent medications: Potential for drug interactions beyond immunosuppressants
Previous tolerance of GLP-1 agonists: If applicable
Reproductive planning: For women of childbearing potential, discussion of contraception requirements and pregnancy planning
If Wegovy is deemed appropriate, your transplant team will establish a monitoring plan that typically includes more frequent assessment of immunosuppressant drug levels (particularly during dose titration), regular monitoring of renal function, and clinical review to assess tolerability and efficacy. Patients should be counselled on recognising adverse effects requiring urgent medical attention, including severe abdominal pain (possible pancreatitis), persistent vomiting affecting medication intake, signs of dehydration, reduced urine output, or any concerns about graft function.
Communication is paramount. Patients should inform their transplant team of all medications, including over-the-counter products and supplements. If Wegovy is prescribed by another clinician (such as a GP or endocrinologist), the transplant team must be informed and involved in ongoing care coordination. Similarly, any healthcare professional considering prescribing Wegovy to a transplant recipient should liaise directly with the transplant centre.
Patients experiencing side effects or concerns whilst taking Wegovy should contact their transplant team promptly rather than discontinuing medication independently, as abrupt changes could affect overall management. For severe symptoms, patients should contact their transplant team urgently, call NHS 111, or attend A&E as appropriate. All suspected adverse reactions should be reported via the MHRA Yellow Card Scheme (yellowcard.mhra.gov.uk).
Wegovy is not officially contraindicated in kidney transplant recipients, but there is very limited clinical data in this population. Safety depends on individual factors including graft stability, time since transplantation, and concurrent medications, requiring careful assessment and monitoring by the transplant team.
Wegovy can potentially affect immunosuppressant absorption through its gastrointestinal side effects (nausea, vomiting, diarrhoea) and delayed gastric emptying. Therapeutic drug monitoring of immunosuppressant levels is advisable when initiating or titrating Wegovy, particularly for medications with narrow therapeutic indices such as tacrolimus.
Kidney transplant recipients must obtain explicit approval from their transplant team before starting Wegovy. The team will assess graft function stability, time since transplantation, immunosuppression regimen, comorbidities, and establish an appropriate monitoring plan including more frequent immunosuppressant level checks and renal function assessment.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.