
Can Wegovy cause stomach ulcers? This is a common concern among patients prescribed semaglutide for weight management. Wegovy (semaglutide 2.4 mg) is a GLP-1 receptor agonist licensed in the UK for obesity treatment, working by slowing gastric emptying and reducing appetite. Whilst gastrointestinal side effects such as nausea and abdominal discomfort are frequently reported, current evidence does not establish a direct causal link between Wegovy and peptic ulcer formation. Understanding the distinction between expected medication effects and symptoms requiring medical evaluation is essential for safe use. This article examines the evidence, explores gastrointestinal risks, and provides guidance on when to seek medical advice.
Quick Answer: Current clinical evidence does not establish a direct causal link between Wegovy (semaglutide) and the development of stomach ulcers.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereWegovy (semaglutide 2.4 mg) is a prescription medicine licensed in the UK for weight management in adults with a BMI ≥30 kg/m² or ≥27 kg/m² with at least one weight-related comorbidity. It is used as an adjunct to reduced-calorie diet and increased physical activity. Wegovy belongs to a class of medications called glucagon-like peptide-1 (GLP-1) receptor agonists, which were originally developed for type 2 diabetes management but have demonstrated significant efficacy in promoting weight loss.
The mechanism of action centres on mimicking the naturally occurring hormone GLP-1, which is released by the intestine after eating. Semaglutide works by:
Slowing gastric emptying – food remains in the stomach longer, promoting feelings of fullness
Reducing appetite – acting on brain receptors that regulate hunger and satiety
Improving insulin secretion – enhancing glucose-dependent insulin release from the pancreas
Decreasing glucagon secretion – reducing glucose production by the liver
Wegovy is administered as a once-weekly subcutaneous injection, with doses gradually increased over 16–20 weeks to reach the maintenance dose of 2.4 mg. This titration schedule helps minimise gastrointestinal side effects, which are the most commonly reported adverse reactions with GLP-1 receptor agonists.
The STEP (Semaglutide Treatment Effect in People with obesity) clinical trials demonstrated average weight loss of 12–15% of body weight over 68 weeks when combined with lifestyle modifications, leading to approval by the Medicines and Healthcare products Regulatory Agency (MHRA).
Importantly, Wegovy is contraindicated during pregnancy and breastfeeding, and is not recommended for use in people under 18 years of age. Understanding how Wegovy interacts with the digestive system is essential for patients and healthcare professionals to recognise potential side effects and distinguish between expected reactions and more serious complications.
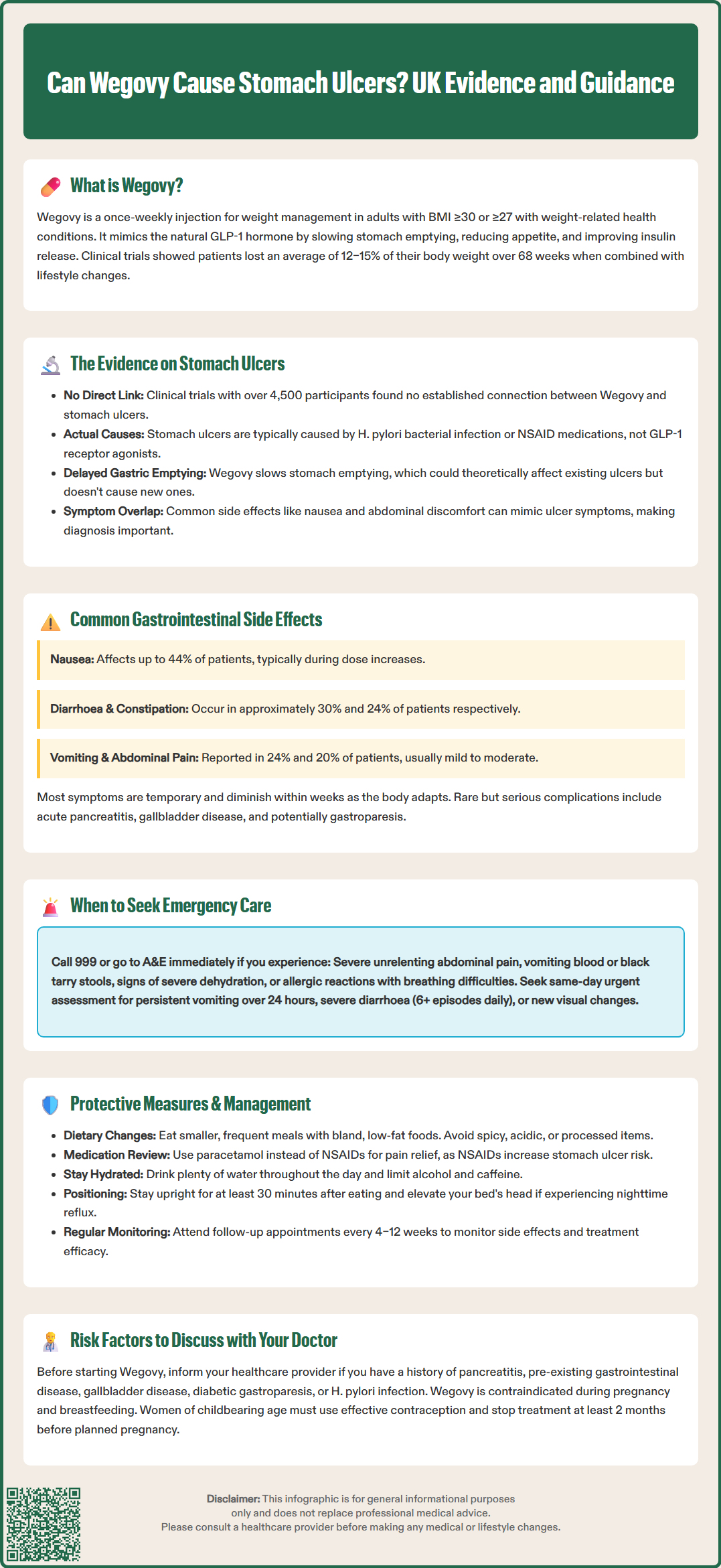
There is currently no established direct causal link between Wegovy and the development of peptic (stomach) ulcers based on available clinical trial data and post-marketing surveillance. Stomach ulcers, medically termed gastric ulcers, are breaks in the protective mucosal lining of the stomach, typically caused by Helicobacter pylori infection or non-steroidal anti-inflammatory drugs (NSAIDs).
The pivotal STEP (Semaglutide Treatment Effect in People with obesity) clinical trials, which included over 4,500 participants, did not identify peptic ulcer disease as a significant adverse event associated with semaglutide. The Summary of Product Characteristics (SmPC) for Wegovy does not list gastric ulcers among known side effects. However, it is important to note that:
Delayed gastric emptying caused by Wegovy could theoretically affect existing ulcers or alter how ulcer-related symptoms present
Nausea and vomiting, common with Wegovy, might mask or mimic ulcer symptoms
Post-marketing surveillance continues to monitor for rare or delayed adverse effects not detected in clinical trials
Some patients have reported upper abdominal discomfort whilst taking GLP-1 receptor agonists, which can sometimes be confused with ulcer-related pain. It is crucial to distinguish between the expected gastrointestinal side effects of Wegovy—which typically improve over time—and symptoms that might indicate a peptic ulcer or other serious gastric pathology.
In the UK, NICE Clinical Guideline 184 recommends that persistent dyspepsia should be investigated with H. pylori testing and a trial of proton pump inhibitor therapy. If you develop persistent or severe upper abdominal pain, particularly if accompanied by blood in vomit or black, tarry stools, you should seek medical evaluation promptly, as these could indicate complications requiring urgent investigation regardless of Wegovy use. NICE Guideline NG12 outlines specific red flag symptoms that warrant urgent referral to rule out upper gastrointestinal malignancy.
Gastrointestinal adverse effects are the most frequently reported side effects with Wegovy, affecting a substantial proportion of users, particularly during dose escalation. Understanding these expected reactions helps patients differentiate between typical medication effects and potentially serious complications.
Common gastrointestinal side effects include:
Nausea (affecting up to 44% of patients in clinical trials)
Diarrhoea (approximately 30% of users)
Vomiting (24% of participants)
Constipation (around 24%)
Abdominal pain (20% of patients)
Dyspepsia (indigestion, affecting 9%)
Gastro-oesophageal reflux disease (GORD)
Flatulence and bloating
These effects typically emerge or intensify when doses are increased and often diminish over several weeks as the body adapts to the medication. The gradual dose titration schedule is specifically designed to minimise these reactions. Most gastrointestinal symptoms are mild to moderate in severity and resolve without requiring treatment discontinuation.
More serious, though rare, gastrointestinal complications have been reported with GLP-1 receptor agonists, including:
Acute pancreatitis – characterised by severe, persistent abdominal pain radiating to the back
Gallbladder disease – including cholecystitis and cholelithiasis
Gastroparesis – severe delayed gastric emptying (though causality remains debated)
There have been some post-marketing case reports of intestinal obstruction with GLP-1 receptor agonists, though this is not currently listed as an established adverse effect in the UK SmPC for Wegovy.
Significant vomiting or diarrhoea can lead to dehydration, which may increase the risk of acute kidney injury, particularly in patients with pre-existing renal impairment. Maintaining adequate hydration is therefore important.
The MHRA and European Medicines Agency (EMA) continue to monitor safety data. Patients should report any unexpected or severe symptoms through the Yellow Card Scheme (yellowcard.mhra.gov.uk), contributing to ongoing pharmacovigilance efforts that help identify rare adverse effects not apparent in pre-marketing trials.
Certain patient characteristics and pre-existing conditions may increase the likelihood of experiencing gastrointestinal complications whilst taking Wegovy. Healthcare professionals should conduct thorough assessments before prescribing, and patients should be aware of relevant risk factors.
Key risk factors include:
History of pancreatitis – caution is advised in patients with previous pancreatitis
Pre-existing gastrointestinal disease – particularly severe gastroparesis or severe GORD
Gallbladder disease – rapid weight loss itself increases gallstone risk
Concurrent medications – particularly NSAIDs, anticoagulants, or other drugs affecting gastric mucosa
Helicobacter pylori infection – if present, may increase ulcer risk independently
Diabetic gastroparesis – in patients with diabetes, pre-existing delayed gastric emptying may be exacerbated
It should be noted that animal studies have shown an increased incidence of thyroid C-cell tumours with semaglutide, though the clinical relevance to humans is unknown. The UK SmPC does not list a contraindication related to medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2.
Wegovy is contraindicated during pregnancy and breastfeeding. Women of childbearing potential should use effective contraception when taking Wegovy and discontinue treatment at least 2 months before a planned pregnancy due to the long half-life of semaglutide.
Warning signs requiring prompt medical evaluation include:
Severe or persistent abdominal pain – especially if sudden onset or radiating to the back
Haematemesis – vomiting blood or 'coffee-ground' material
Melaena – black, tarry stools indicating upper gastrointestinal bleeding
Persistent vomiting – preventing adequate fluid or medication intake
Signs of dehydration – reduced urination, dizziness, dry mouth
Unexplained weight loss beyond expected therapeutic effect
Dysphagia – difficulty swallowing
Jaundice – yellowing of skin or eyes, suggesting gallbladder or liver involvement
Patients should maintain regular follow-up appointments, typically scheduled at 4–12 week intervals initially, allowing healthcare professionals to monitor tolerance, efficacy, and potential adverse effects. Blood tests may be indicated if symptoms suggest pancreatitis (amylase/lipase), renal impairment, or other complications.
Knowing when gastrointestinal symptoms require professional evaluation is crucial for patient safety. Whilst mild nausea or altered bowel habits are expected with Wegovy, certain presentations warrant urgent or routine medical assessment.
Seek emergency medical attention (call 999 or attend A&E) if you experience:
Severe, unrelenting abdominal pain – particularly if accompanied by fever or vomiting
Signs of gastrointestinal bleeding – vomiting blood, passing black stools, or feeling faint
Symptoms of severe dehydration – inability to keep fluids down, reduced consciousness, or minimal urine output
Suspected allergic reaction – facial swelling, difficulty breathing, or widespread rash
Seek urgent same-day assessment if you develop:
New visual changes – particularly if you have diabetes or pre-existing retinopathy (contact your GP, diabetes team or eye casualty)
Persistent vomiting lasting more than 24 hours
Severe diarrhoea – more than six episodes daily or lasting beyond 48 hours
Unremitting nausea preventing normal eating or drinking
New or worsening abdominal pain that differs from initial side effects
Contact your GP or prescribing clinician within 24–48 hours if you develop:
Symptoms of gallbladder disease – right upper quadrant pain, especially after eating fatty foods
Unexplained fever with abdominal symptoms
Difficulty taking other essential medications due to gastrointestinal symptoms
Persistent dyspepsia despite simple measures
Routine follow-up is appropriate for:
Mild, manageable nausea or altered bowel habits
Questions about dose titration or administration technique
Discussion of symptom management strategies
Scheduled monitoring appointments
NICE guidance (TA875) on obesity management emphasises the importance of regular clinical review for patients on pharmacological weight management treatments. Your prescriber should assess treatment response, tolerability, and any adverse effects at predetermined intervals, typically adjusting management plans accordingly. Never discontinue Wegovy abruptly without medical guidance, particularly if you have diabetes, as this may affect glycaemic control.
Whilst Wegovy does not directly cause stomach ulcers, implementing strategies to minimise gastrointestinal discomfort and protect digestive health can improve treatment tolerance and outcomes.
Dietary modifications to reduce symptoms:
Eat smaller, more frequent meals – rather than three large meals, which can overwhelm delayed gastric emptying
Choose bland, low-fat foods – particularly during dose escalation periods
Avoid trigger foods – spicy, acidic, or heavily processed items that may exacerbate nausea
Stay well hydrated – sipping water throughout the day, especially if experiencing vomiting or diarrhoea
Limit alcohol and caffeine – both can irritate the gastric lining and worsen reflux
Eat slowly and chew thoroughly – supporting the digestive process
Medication management considerations:
Avoid NSAIDs when possible – paracetamol is preferable for pain relief, as NSAIDs increase ulcer risk
Review all medications – with your pharmacist or GP to identify potential interactions
Take Wegovy consistently – same day each week, with or without food as preferred
Consider timing of other medications – while delayed gastric emptying generally has minimal effect on drug absorption, monitoring may be needed for medications with a narrow therapeutic index
Lifestyle measures:
Remain upright after eating – for at least 30 minutes to reduce reflux
Elevate the head of your bed – if experiencing nocturnal reflux symptoms
Manage stress – which can exacerbate gastrointestinal symptoms
Maintain physical activity – as tolerated, supporting overall digestive function
When symptoms persist despite these measures, discuss with your healthcare provider who may recommend:
Domperidone has restricted use due to cardiac risks and should be avoided in certain conditions
Proton pump inhibitors – if significant reflux or dyspepsia develops, following NICE guidance (CG184)
Dose adjustment – temporarily reducing or maintaining current dose before further escalation
Treatment interruption – in cases of intolerable symptoms
Regular communication with your healthcare team ensures optimal management of both weight loss goals and treatment tolerability, maximising the benefits of Wegovy whilst minimising adverse effects.
No, current clinical evidence does not establish a direct causal link between Wegovy and peptic ulcer formation. Stomach ulcers are typically caused by Helicobacter pylori infection or NSAID use, not by GLP-1 receptor agonists like semaglutide.
The most common gastrointestinal side effects include nausea (up to 44% of patients), diarrhoea (30%), vomiting (24%), constipation (24%), and abdominal pain (20%). These symptoms typically emerge during dose escalation and often diminish over several weeks.
Seek emergency care immediately if you experience severe unrelenting abdominal pain, vomit blood, pass black stools, or develop signs of severe dehydration. Contact your GP urgently for persistent vomiting lasting over 24 hours, severe diarrhoea, or new abdominal pain that differs from initial side effects.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.