
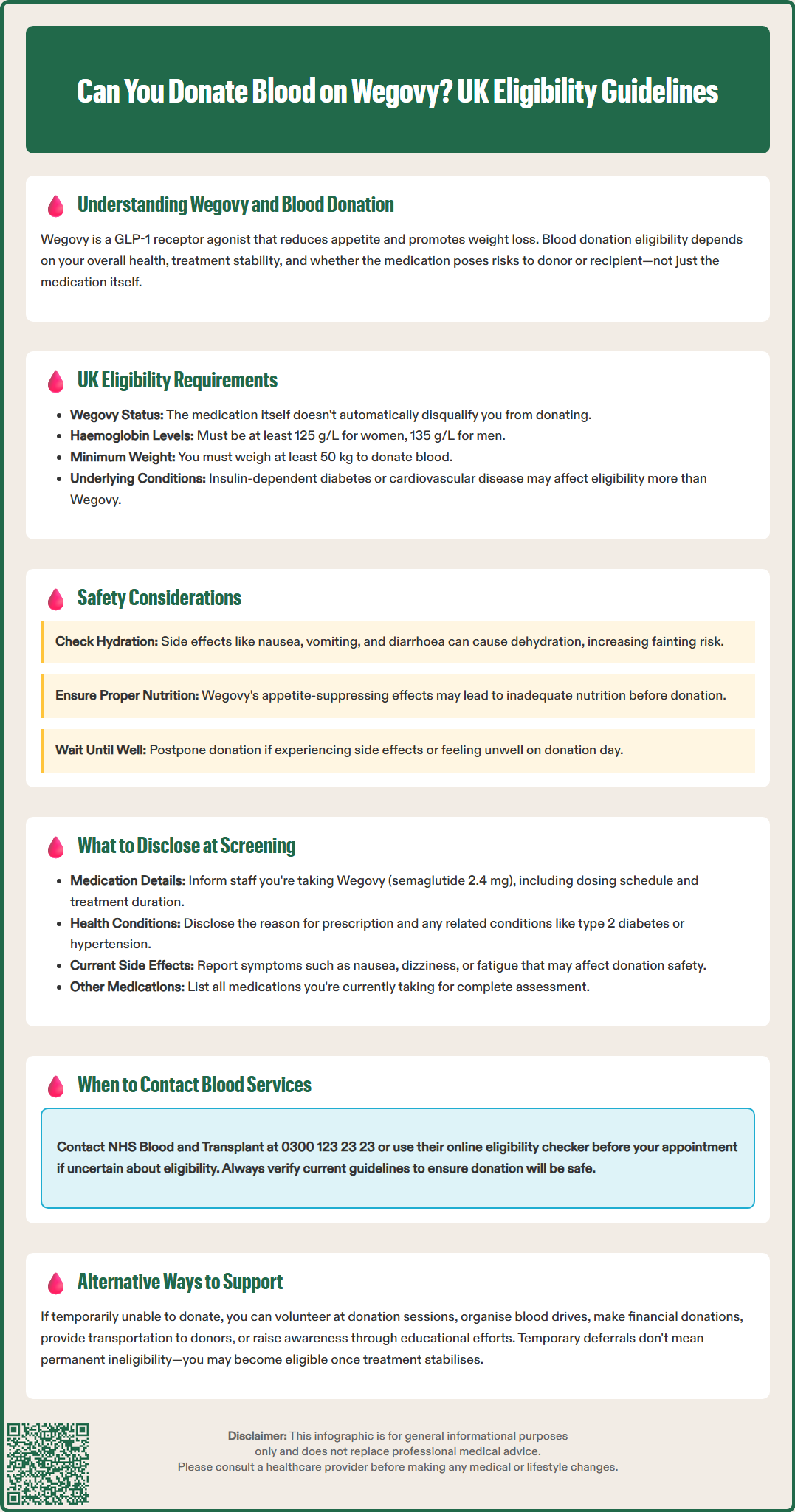
Many patients prescribed Wegovy (semaglutide 2.4 mg) for weight management wonder whether they can continue donating blood during treatment. Wegovy, a GLP-1 receptor agonist approved for chronic weight management, works by regulating appetite and reducing caloric intake. Blood donation eligibility whilst taking Wegovy depends on your overall health, treatment stability, and whether you're experiencing medication side effects. NHS Blood and Transplant (NHSBT) evaluates donors individually, considering underlying conditions, haemoglobin levels, blood pressure, and weight requirements. This article explains UK blood donation guidelines for patients taking Wegovy, safety considerations, and what information to disclose during pre-donation screening.
Quick Answer: You may be able to donate blood whilst taking Wegovy if you meet NHSBT eligibility criteria, are otherwise well, and not experiencing significant side effects.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereWegovy (semaglutide 2.4 mg) is a prescription medication approved for chronic weight management in adults with obesity or overweight with weight-related comorbidities. As a glucagon-like peptide-1 (GLP-1) receptor agonist, it works by mimicking a naturally occurring hormone that regulates appetite and food intake, leading to reduced caloric consumption and sustained weight loss.
Many patients taking Wegovy wonder whether they can continue donating blood while on this medication. Blood donation eligibility depends on multiple factors, including the donor's overall health, the medication being taken, and whether that medication could pose risks to either the donor or the recipient. The primary concern for blood services is ensuring the safety of both parties involved in the donation process.
According to NHS Blood and Transplant (NHSBT) guidance, most medications do not automatically disqualify donors, but the underlying conditions being treated often determine eligibility. For example, people who use insulin for diabetes cannot donate blood, while those managing diabetes with diet or non-insulin tablets may be eligible if they have no complications such as retinopathy, nephropathy, neuropathy, or cardiovascular disease.
The decision to donate blood while taking Wegovy should involve consideration of your treatment stability, blood sugar control (if you have diabetes), cardiovascular health, and whether you're experiencing any side effects from the medication. Patients should always disclose all medications during the pre-donation screening process, as this allows trained staff to make informed decisions about donation eligibility based on current NHSBT and JPAC guidelines.
NHS Blood and Transplant (NHSBT) maintains comprehensive guidelines regarding medication use and blood donation eligibility. While Wegovy itself is not specifically listed as a disqualifying medication, the organisation evaluates donors based on their overall health profile and the reason for taking the medication.
Key considerations for UK donors taking Wegovy include:
Underlying health conditions: If you're taking Wegovy for obesity-related conditions, these may affect your eligibility more than the medication itself. For example, people with diabetes requiring insulin cannot donate, while those with well-controlled hypertension generally can. Many cardiovascular conditions (such as angina, heart failure, or previous heart attack) may result in deferral.
Haemoglobin levels: Donors must meet minimum haemoglobin thresholds — 125 g/L for women and 135 g/L for men — as specified by NHSBT. Weight-loss medications don't typically affect these levels, but nutritional status during weight loss might.
Blood pressure: Well-controlled hypertension is generally acceptable for donation, while uncontrolled hypertension may result in deferral.
Weight requirements: Donors must weigh at least 50 kg (7 stone 12 pounds), according to NHSBT. While taking Wegovy for weight loss, ensure you maintain this minimum threshold.
Age criteria: First-time donors should be 17–65 years old, and returning donors may donate up to age 70 or older if otherwise eligible.
NHSBT recommends that potential donors contact their helpline (0300 123 23 23) or use the online eligibility checker before attending a donation session. The pre-donation health screening includes questions about all medications, and trained staff will assess whether donation is appropriate on that particular day. If you're experiencing side effects from Wegovy — such as nausea, vomiting, diarrhoea, dehydration, or fatigue — you should defer donation until you feel fully well, in line with NHSBT and JPAC guidance.
Semaglutide's pharmacological properties are important to understand when considering blood donation. As a peptide medication with a molecular weight of approximately 4,100 Daltons, semaglutide has a half-life of about one week, meaning it remains in the system for an extended period. The medication is administered subcutaneously once weekly and reaches steady-state concentrations after 4–5 weeks of treatment, according to the Wegovy Summary of Product Characteristics (SmPC).
From a transfusion safety perspective, semaglutide is not listed on NHSBT or JPAC deferral lists, and eligibility is assessed on a case-by-case basis. Current UK guidance indicates that weight-loss medicines, including injectables like Wegovy, are acceptable if the donor is otherwise well and not experiencing significant side effects on the day of donation.
Donor safety considerations are more relevant when taking Wegovy. According to the SmPC, semaglutide can cause:
Gastrointestinal side effects: Nausea, vomiting, and diarrhoea may lead to dehydration, which increases the risk of donation-related adverse events such as fainting or dizziness.
Dizziness: Some patients experience dizziness, which could be exacerbated by blood donation.
Reduced food intake: The appetite-suppressing effects might mean donors have not consumed adequate nutrition before donation.
Blood services prioritise donor wellbeing, and individuals experiencing active side effects from Wegovy should wait until they feel well before attempting to donate. Adequate hydration, proper nutrition, and stable medication tolerance are essential prerequisites for safe blood donation while taking semaglutide. NHSBT recommends eating and drinking well before donation to minimise the risk of feeling faint.
Transparency during the pre-donation screening process is essential for your safety and the safety of blood recipients. When attending a blood donation appointment while taking Wegovy, you should provide comprehensive information to the screening staff.
Information to disclose includes:
Medication name and dose: Specify that you're taking Wegovy (semaglutide 2.4 mg) and your current dosing schedule.
Duration of treatment: How long you've been taking the medication and whether you've reached a stable maintenance dose.
Reason for prescription: Explain that Wegovy is prescribed for weight management, and mention any related conditions such as type 2 diabetes, hypertension, or cardiovascular disease.
Current side effects: Report any ongoing symptoms, particularly nausea, vomiting, diarrhoea, dizziness, or fatigue.
Recent weight changes: Significant weight loss might affect your eligibility if you're approaching the minimum weight threshold.
Other medications: List all other prescription and over-the-counter medications you're taking.
The screening staff will assess whether donation is appropriate based on this information combined with your vital signs, haemoglobin level, and overall presentation. Be prepared to answer questions about your eating and drinking habits that day, as adequate nutrition and hydration are particularly important for donors taking appetite-suppressing medications.
If you're uncertain about your eligibility, contact the blood service before your appointment. NHS Blood and Transplant operates a donor helpline (0300 123 23 23) and provides an online eligibility checker on their website. This proactive approach saves time and ensures you only attend when donation is likely to be safe and successful.
If you're temporarily unable to donate blood while taking Wegovy — whether due to side effects, weight considerations, or other health factors — there are numerous alternative ways to support blood services and help patients in need.
Volunteer opportunities include:
Donation session assistance: Help staff at blood donation venues with registration, refreshments, or donor support.
Community outreach: Organise blood drives at your workplace, place of worship, or community centre.
Social media advocacy: Share information about the importance of blood donation and encourage eligible friends and family to donate.
Transportation support: Offer rides to donation appointments for those without transport.
Financial contributions to blood services help fund:
Collection and processing equipment.
Donor recruitment campaigns.
Research into blood safety and storage.
Emergency blood supply maintenance.
Educational initiatives are valuable for raising awareness. You might give presentations about the importance of blood donation, share your own donation history (if applicable), or help dispel common myths that prevent people from donating.
Once your treatment stabilises and any side effects resolve, you may be able to donate. Patients taking Wegovy may become eligible once they've adjusted to the medication, maintained a stable weight above the minimum threshold, and are free from troublesome side effects. Regular donors should inform blood services if they start taking Wegovy, but donation may still be possible depending on individual circumstances.
Remember that blood donation eligibility can change over time. Even if you're deferred temporarily, you may be able to donate in the future. Stay engaged with blood services, maintain your health, and continue supporting this vital community service in whatever capacity you can.
No, Wegovy is not listed as a disqualifying medication by NHS Blood and Transplant. Eligibility is assessed individually based on your overall health, underlying conditions, and whether you're experiencing side effects on the day of donation.
Donors must weigh at least 50 kg (7 stone 12 pounds) and meet minimum haemoglobin levels of 125 g/L for women and 135 g/L for men, according to NHSBT guidelines.
Yes, you should defer donation if experiencing active side effects such as nausea, vomiting, diarrhoea, dehydration, dizziness, or fatigue. Wait until you feel fully well before attempting to donate blood.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.