
Rybelsus (semaglutide) is a prescription medicine used to treat type 2 diabetes in adults across the UK. Like all medications, Rybelsus tablets carry an expiry date that guarantees their quality and effectiveness when stored correctly. Many patients wonder whether it is safe to take expired Rybelsus, particularly if they discover their medication has passed its expiry date. This article explains why taking expired Rybelsus is not advisable, outlines the potential risks to diabetes control, provides guidance on proper storage to maintain tablet stability, and explains how to obtain a replacement prescription through the NHS if your medication has expired.
Quick Answer: No, you should not take expired Rybelsus, as the medication may have degraded and cannot be guaranteed to remain effective or safe for managing type 2 diabetes.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereRybelsus (semaglutide) is an oral glucagon-like peptide-1 (GLP-1) receptor agonist licensed in the UK for the treatment of type 2 diabetes mellitus in adults. As with all prescription medicines, Rybelsus tablets carry an expiry date printed on the packaging, which indicates the period during which the manufacturer guarantees the medicine's quality and stability when stored correctly.
Expiry dates are determined through rigorous stability testing conducted during the drug development process. These studies assess how environmental factors—such as temperature, humidity, and light exposure—affect the active pharmaceutical ingredient (semaglutide) and the tablet formulation over time. The Medicines and Healthcare products Regulatory Agency (MHRA) requires manufacturers to demonstrate that medicines remain stable and effective until the stated expiry date under recommended storage conditions.
It is not advisable to take expired Rybelsus. Once a medicine passes its expiry date, there is no guarantee that it retains its intended therapeutic effect or remains safe for consumption. The active ingredient may degrade, potentially reducing blood glucose control in people with diabetes. Additionally, the absorption-enhancing excipient sodium N-(8-[2-hydroxybenzoyl] amino) caprylate (SNAC), which facilitates semaglutide absorption in the stomach, may theoretically deteriorate over time, though specific data on this beyond the expiry date are not publicly available.
Patients should always check the expiry date before taking any dose of Rybelsus. If you discover that your medication has expired, do not take it. Instead, contact your GP surgery or community pharmacy for advice on obtaining a replacement prescription. If you have inadvertently taken a single dose of expired Rybelsus, this is unlikely to be harmful, but you should not take additional expired doses, should obtain an in-date supply promptly, and monitor your blood glucose levels. Proper medication management, including regular monitoring of expiry dates, is an essential component of safe and effective diabetes care.
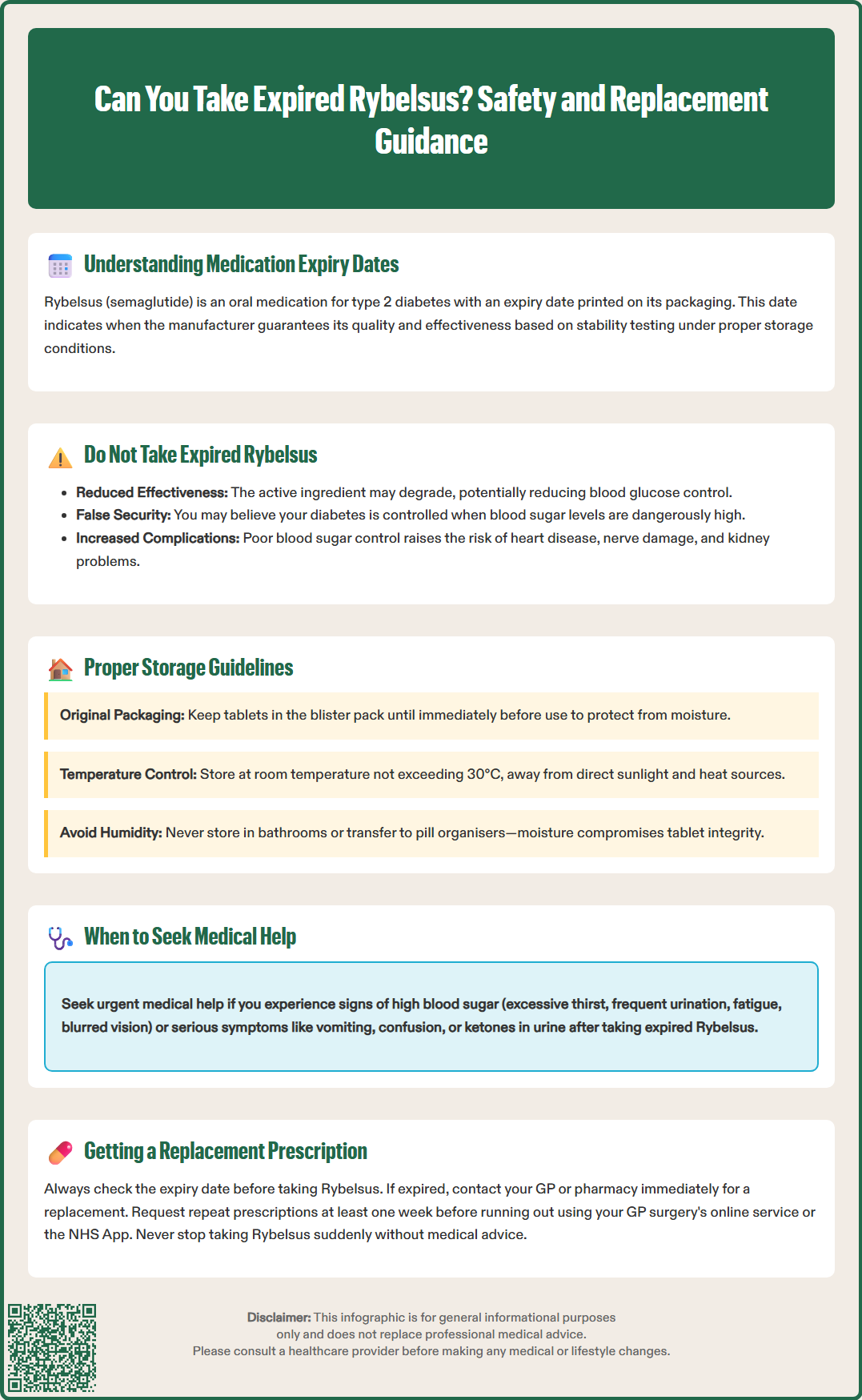
Taking expired Rybelsus poses several potential risks that can compromise both treatment efficacy and patient safety. The primary concern is reduced therapeutic effectiveness. As semaglutide degrades over time, the concentration of active drug in each tablet may fall below the labelled dose (3 mg, 7 mg, or 14 mg). This degradation can result in suboptimal glycaemic control, leading to elevated blood glucose levels (hyperglycaemia) and potentially increasing the risk of diabetes-related complications such as cardiovascular disease, neuropathy, nephropathy, and retinopathy.
Whilst there is no official evidence suggesting that expired Rybelsus becomes acutely toxic, chemical degradation of pharmaceutical ingredients is a general theoretical concern for medicines. Although this risk is generally considered low for most medications, including Rybelsus, and there are no specific data indicating toxicity of degraded semaglutide tablets.
Another significant risk is the false sense of security that taking expired medication may provide. Patients might believe they are adequately managing their diabetes when, in reality, their blood glucose levels are poorly controlled due to reduced drug potency. This can delay appropriate medical intervention and adjustment of treatment regimens.
Regulatory and safety perspective: The MHRA and NHS guidance consistently advise against using any medicine past its expiry date. Healthcare professionals cannot guarantee the safety or efficacy of expired medications, and their use falls outside the terms of the product licence.
If you experience symptoms of persistent hyperglycaemia such as excessive thirst, frequent urination, fatigue, blurred vision, or blood glucose readings consistently above 20 mmol/L, seek urgent medical advice. If you develop vomiting, drowsiness, confusion, or have positive ketones in your urine, contact your GP immediately or call NHS 111 if out of hours. These could be signs of a more serious condition requiring prompt treatment.
If you suspect any adverse effects from taking Rybelsus (expired or in-date), report them through the MHRA Yellow Card scheme at yellowcard.mhra.gov.uk or via the Yellow Card app.
Proper storage of Rybelsus is essential to maintain its stability, potency, and safety throughout its shelf life. According to the Summary of Product Characteristics (SmPC) approved by the MHRA, Rybelsus tablets should be kept in the original blister pack to protect them from moisture and should not be removed until just before use. The medication does not require refrigeration and should be kept at room temperature, not above 30°C.
Key storage recommendations include:
Keep tablets in the original packaging until immediately before use. The blister pack provides a protective barrier against moisture, which can degrade the tablet formulation.
Store in a cool, dry place away from direct sunlight and heat sources such as radiators, windowsills, or kitchen appliances.
Avoid bathroom storage, as the humidity from showers and baths can compromise tablet integrity, even within sealed blisters.
Keep out of reach and sight of children to prevent accidental ingestion.
Do not transfer tablets to pill organisers or alternative containers, as this exposes them to environmental moisture and makes tracking expiry dates more difficult.
Do not use tablets from damaged blisters, as the protective seal may be compromised.
When travelling, avoid leaving medication in cars or other environments that may become hot, as temperatures can exceed 30°C.
Checking your medication regularly is an important habit. When you collect a new prescription, note the expiry date and ensure you will use the medication before it expires. If you have multiple packs, use the one with the earliest expiry date first (first-in, first-out principle).
If you notice any visible changes to your Rybelsus tablets—such as discolouration, crumbling, or an unusual odour—do not take them, even if they are within the expiry date. Return them to your community pharmacy for safe disposal and advice. Pharmacists can assess whether the medication has been compromised and arrange a replacement if necessary. Proper storage practices, combined with regular medication reviews, help ensure that Rybelsus remains effective in managing your type 2 diabetes.
If your Rybelsus has expired or is approaching its expiry date, obtaining a new prescription through the NHS is straightforward. Rybelsus is a prescription-only medicine (POM) in the UK, meaning it can only be supplied by a registered pharmacy with a valid prescription from an appropriate prescriber, typically your GP or diabetes specialist.
Steps to obtain a new prescription:
Contact your GP surgery in advance—ideally at least one week before your current supply runs out. Many surgeries offer online prescription request services through their website or the NHS App, which can be more convenient than telephone requests.
Request a repeat prescription if Rybelsus is already listed on your repeat medication list. Your GP may issue this without requiring an appointment, though periodic medication reviews are necessary.
Attend medication reviews as requested by your GP or practice nurse. NICE guideline NG28 recommends regular monitoring of HbA1c levels (typically every 3–6 months until stable, then 6-monthly) and assessment of treatment response, tolerability, and cardiovascular risk factors for patients taking GLP-1 receptor agonists.
Collect your prescription from your nominated community pharmacy. Some surgeries send prescriptions electronically directly to your chosen pharmacy through the Electronic Prescription Service (EPS), eliminating the need to collect a paper prescription.
If you have run out of medication, contact your GP surgery as soon as possible. Explain the situation, and they may be able to issue an emergency prescription or arrange a same-day appointment if clinically appropriate. Community pharmacists may also be able to provide an emergency supply of Rybelsus (subject to professional assessment and eligibility). If you need help outside of normal surgery hours, contact NHS 111 for advice on accessing medication.
Do not stop taking Rybelsus abruptly without medical advice, as this may lead to deterioration in glycaemic control.
NICE recommendations support the use of oral semaglutide (Rybelsus) as a treatment option for type 2 diabetes in specific circumstances, typically when other treatments have not achieved adequate control. Your prescriber will ensure that Rybelsus remains the most appropriate treatment for your individual circumstances during regular reviews. If you experience any difficulties accessing your medication or have concerns about treatment, your diabetes specialist nurse or GP can provide guidance and support.
Taking a single dose of expired Rybelsus is unlikely to cause harm, but do not take additional expired doses. Contact your GP surgery or pharmacy immediately to obtain an in-date supply and monitor your blood glucose levels closely.
Return expired Rybelsus tablets to your community pharmacy for safe disposal. Do not throw them in household waste or flush them down the toilet, as this can harm the environment.
Community pharmacists may be able to provide an emergency supply of Rybelsus subject to professional assessment and eligibility criteria. Contact your pharmacy to discuss your situation if you have run out of in-date medication.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.