
Combining weight-loss medications is an increasingly common query amongst patients seeking enhanced results. Phentermine, a sympathomimetic appetite suppressant, and Wegovy (semaglutide), a GLP-1 receptor agonist, work through entirely different mechanisms. Whilst both are used for weight management, their concurrent use raises important safety and efficacy questions. Currently, there is no official UK guidance approving this combination, and phentermine is not licensed in the UK. This article examines the evidence, potential risks, and professional recommendations regarding the combined use of phentermine with Wegovy, alongside safer alternative approaches to weight management.
Quick Answer: Combining phentermine with Wegovy is generally not recommended without specialist medical supervision, as there is no official UK guidance approving this combination and the safety profile remains inadequately studied.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HerePhentermine and Wegovy (semaglutide) represent two distinct pharmacological approaches to weight management, each working through fundamentally different mechanisms within the body.
Phentermine is a sympathomimetic amine that functions as an appetite suppressant. It acts primarily on the central nervous system by stimulating the release of noradrenaline and, to a lesser extent, dopamine and serotonin. This neurotransmitter activity reduces hunger signals and creates a feeling of satiety. Phentermine is chemically related to amphetamines and is classified as a controlled substance in many jurisdictions due to its stimulant properties. It increases heart rate and blood pressure whilst suppressing appetite, making it suitable only for short-term use—typically 12 weeks or less. The medication is generally prescribed for individuals with a body mass index (BMI) of 30 kg/m² or above, or 27 kg/m² with weight-related comorbidities. Importantly, phentermine is not currently licensed in the UK and is only available via named-patient import.
Wegovy (semaglutide 2.4 mg) belongs to an entirely different drug class known as glucagon-like peptide-1 (GLP-1) receptor agonists. Originally developed for type 2 diabetes management, semaglutide mimics the action of the naturally occurring GLP-1 hormone. It works by slowing gastric emptying, enhancing feelings of fullness after meals, and acting on appetite centres in the brain to reduce hunger. Additionally, it improves glycaemic control by stimulating insulin secretion in a glucose-dependent manner. Wegovy is administered as a once-weekly subcutaneous injection and is licensed for long-term weight management. Unlike phentermine, it does not have stimulant properties and works through metabolic and hormonal pathways rather than direct central nervous system stimulation.
These contrasting mechanisms raise important questions about whether combining these medications is safe or advisable for patients seeking enhanced weight loss outcomes.
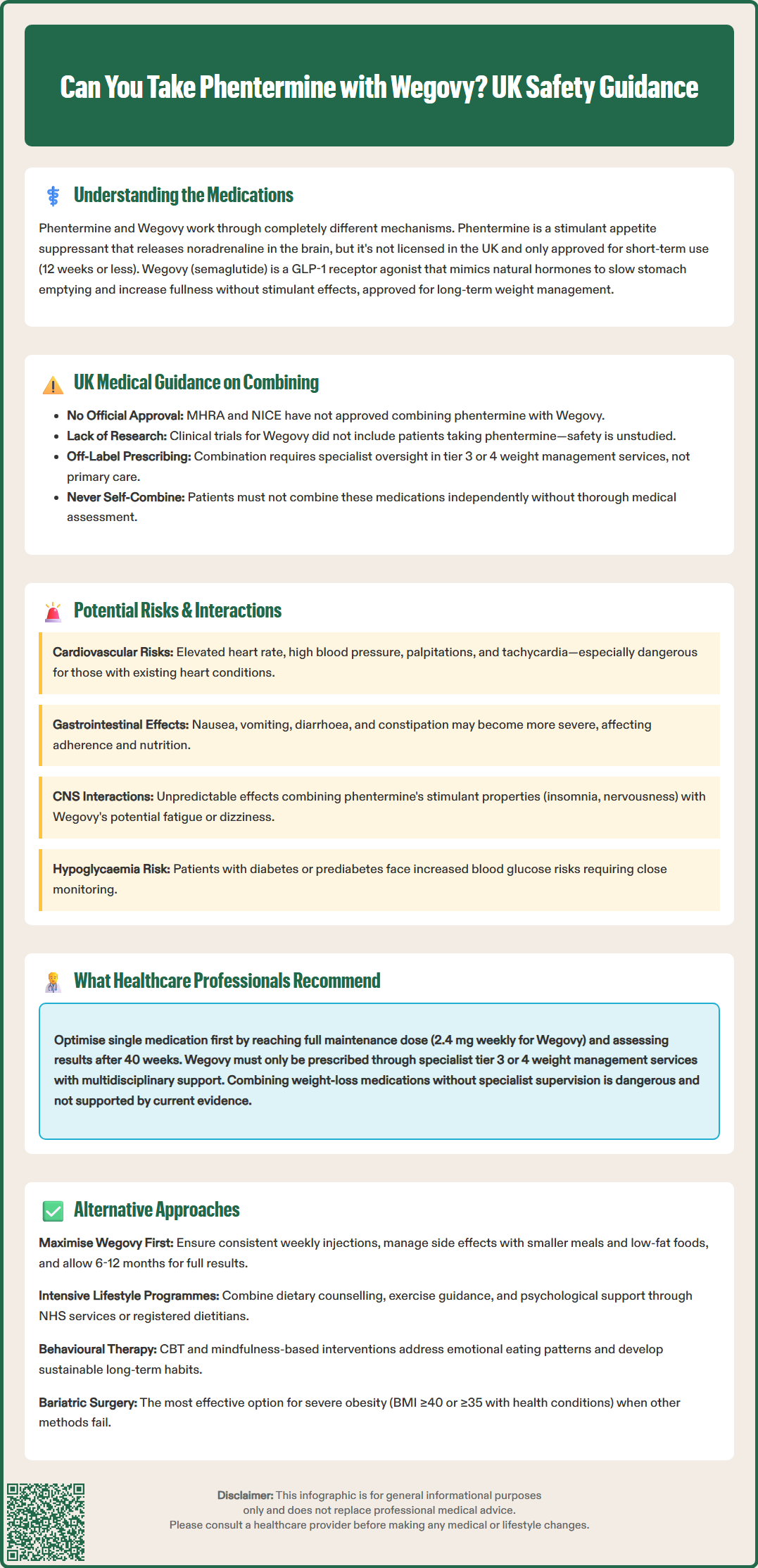
The concurrent use of phentermine and Wegovy has not been extensively studied in clinical trials, and there is currently no official guidance from UK regulatory bodies such as the MHRA (Medicines and Healthcare products Regulatory Agency) or NICE (National Institute for Health and Care Excellence) specifically approving this combination. The prescribing information for both medications does not explicitly address their combined use, which means healthcare professionals must exercise clinical judgement when considering such combinations.
In clinical practice, the combination of these two weight-loss medications is generally not recommended without compelling clinical justification and close medical supervision. The pivotal trials that established Wegovy's efficacy (the STEP programme) did not include participants taking phentermine concurrently, meaning the safety profile and potential interactions of this combination remain inadequately characterised. Similarly, phentermine's clinical trials were conducted as monotherapy or occasionally with topiramate (as in the combination product Qsymia, not licensed in the UK).
It is important to understand that combining these medications would constitute off-label prescribing in the UK, requiring informed consent and specialist oversight. Additionally, NICE Technology Appraisal 875 (TA875) specifies that Wegovy should only be prescribed within specialist tier 3 or 4 weight management services, not in primary care.
Some healthcare providers in specialist weight management services may occasionally consider combining these agents in carefully selected patients who have not achieved adequate weight loss with monotherapy. However, this would typically occur only in tertiary care settings with appropriate monitoring protocols in place. The decision to combine these medications should never be made by patients independently; it requires thorough medical assessment, consideration of individual risk factors, and ongoing clinical oversight.
For most patients, the standard approach involves optimising one medication at a time, ensuring adequate lifestyle modifications are in place, and monitoring response before considering any changes to the treatment regimen. The NHS weight management pathway typically emphasises a stepwise approach, beginning with lifestyle interventions and progressing to pharmacotherapy only when appropriate, with regular review of treatment efficacy and tolerability.
Combining phentermine with Wegovy introduces several theoretical and practical safety concerns that warrant careful consideration.
Cardiovascular effects represent a primary concern. Phentermine increases sympathetic nervous system activity, leading to elevated heart rate and blood pressure. Whilst Wegovy does not typically cause these effects directly, the combination could potentially amplify cardiovascular strain, particularly in patients with pre-existing hypertension, coronary artery disease, or arrhythmias. Phentermine is contraindicated in patients with cardiovascular disease, and adding it to Wegovy therapy could increase the risk of adverse cardiac events including palpitations, tachycardia, and elevated blood pressure. Historically, phentermine in combination with fenfluramine (now withdrawn) was associated with valvular heart disease, which underscores the need for caution with novel combinations.
Gastrointestinal side effects may be compounded when these medications are used together. Wegovy commonly causes nausea, vomiting, diarrhoea, and constipation, particularly during dose escalation. Phentermine can also cause gastrointestinal disturbances, including dry mouth and constipation. The combined effect might result in more severe or prolonged gastrointestinal symptoms, potentially affecting medication adherence and nutritional status.
Central nervous system effects require consideration as well. Phentermine's stimulant properties can cause insomnia, nervousness, restlessness, and mood changes. Whilst Wegovy does not have direct CNS stimulant effects, some patients report fatigue or dizziness. The interaction between these different neurological effects is unpredictable and could potentially affect mental wellbeing or daily functioning.
Hypoglycaemia risk may be relevant for patients with diabetes or prediabetes. Wegovy enhances insulin secretion and improves glycaemic control, whilst phentermine's effects on blood glucose are less predictable. Patients taking diabetes medications alongside this combination would require particularly close glucose monitoring.
There is also the consideration of additive costs and medication burden. Both medications are expensive, and combining them may not be cost-effective if the additional benefit is marginal. Furthermore, managing two weight-loss medications simultaneously increases complexity for both patients and healthcare providers, potentially affecting adherence and monitoring quality.
Patients and healthcare professionals are encouraged to report any suspected adverse reactions to medicines via the MHRA Yellow Card Scheme (yellowcard.mhra.gov.uk), which is vital for ongoing safety monitoring.
Healthcare professionals in the UK typically advise a cautious, evidence-based approach to weight management pharmacotherapy, prioritising patient safety and established clinical guidelines.
Monotherapy optimisation is the preferred first-line strategy. Before considering any combination therapy, clinicians recommend ensuring that a single agent is used at its optimal dose with adequate duration of treatment. For Wegovy, this means completing the dose escalation schedule to reach the maintenance dose of 2.4 mg weekly, provided it is tolerated. According to NICE TA875, treatment response should be assessed after 40 weeks; if patients have not achieved at least 5% weight loss, discontinuation should be considered.
Specialist service involvement is essential for pharmacological weight management in the UK. NICE guidance stipulates that Wegovy should only be prescribed within specialist tier 3 or 4 weight management services with multidisciplinary team support, not in primary care. These services can provide comprehensive assessment, including investigations for secondary causes of obesity (such as hypothyroidism or Cushing's syndrome) and identification of red flags that might warrant further investigation (e.g., unexplained weight loss).
Comprehensive lifestyle intervention remains the foundation of any weight management programme. The Royal College of Physicians and NICE emphasise that pharmacotherapy should always be an adjunct to—not a replacement for—dietary modification, increased physical activity, and behavioural support. Healthcare professionals recommend referral to specialist weight management services, dietitians, or structured programmes that address the multifactorial nature of obesity.
Risk-benefit assessment is crucial when patients enquire about combining medications. Specialists will evaluate individual cardiovascular risk factors, mental health status, medication history, and previous treatment responses. For patients who have tried and failed multiple monotherapy approaches, referral to a specialist obesity service or bariatric medicine clinic may be more appropriate than attempting combination pharmacotherapy without specialist oversight.
Regular monitoring protocols are essential if combination therapy is ever considered. This would include:
Baseline and ongoing cardiovascular assessment (blood pressure, heart rate, ECG if indicated)
Regular weight and BMI measurements
Monitoring for adverse effects, particularly gastrointestinal and cardiovascular symptoms
Assessment of mental health and wellbeing
Review of concomitant medications and potential interactions
Patient education is paramount. Healthcare professionals should ensure patients understand that weight-loss medications are not 'quick fixes' and that sustainable results require long-term lifestyle changes. Patients should be counselled about realistic weight loss expectations, potential side effects, and the importance of reporting any concerning symptoms promptly. They should also understand that combining medications without medical supervision could be dangerous and is not supported by current evidence.
For patients seeking enhanced weight loss beyond what single-agent pharmacotherapy provides, several evidence-based alternatives exist that may be safer and more effective than combining phentermine with Wegovy.
Optimising Wegovy therapy should be the priority. Ensuring adherence to the weekly injection schedule, managing side effects proactively, and allowing sufficient time for the medication to exert its full effect (typically 6–12 months) can maximise outcomes. Some patients may benefit from adjusting the timing of injections or implementing strategies to minimise gastrointestinal side effects, such as eating smaller, more frequent meals and avoiding high-fat foods.
Orlistat is currently the only licensed oral anti-obesity medication in the UK. It works by inhibiting pancreatic lipases, reducing fat absorption from the diet. While its weight loss effects are more modest than Wegovy, it has a well-established safety profile and may be suitable for patients who cannot tolerate or access GLP-1 receptor agonists.
Intensive lifestyle interventions have demonstrated significant efficacy in clinical trials. The NHS Diabetes Prevention Programme and similar structured behavioural programmes combine dietary counselling, physical activity guidance, and psychological support. Evidence shows that intensive lifestyle interventions can achieve 5–10% weight loss in motivated individuals. Referral to a registered dietitian for personalised nutritional guidance, or to an exercise physiologist for tailored physical activity plans, can substantially enhance outcomes.
Psychological support and behavioural therapy address the emotional and behavioural aspects of eating that medications alone cannot resolve. Cognitive behavioural therapy (CBT) for weight management, mindfulness-based interventions, and support groups can help patients develop sustainable eating patterns and coping strategies. Many NHS weight management services incorporate psychological support as a core component.
Consideration of other GLP-1 receptor agonists or future medications may be appropriate. Tirzepatide, a dual GLP-1/GIP receptor agonist, has shown superior weight loss compared to semaglutide in clinical trials, though it is not yet licensed in the UK for weight management. Discussing emerging treatment options with a specialist may provide alternatives to combination therapy.
Bariatric surgery remains the most effective intervention for severe obesity (BMI ≥40 kg/m² or ≥35 kg/m² with comorbidities) when other approaches have been unsuccessful. Procedures such as gastric bypass or sleeve gastrectomy can achieve substantial, sustained weight loss and improvement in obesity-related conditions. NICE recommends considering bariatric surgery for appropriate candidates, and referral to a bariatric surgical service should be discussed with patients who meet the criteria.
Addressing underlying medical conditions that may impair weight loss is also essential. Hypothyroidism, polycystic ovary syndrome, Cushing's syndrome, and certain medications (such as antipsychotics or corticosteroids) can contribute to weight gain or resistance to weight loss. Comprehensive medical evaluation and management of these conditions may improve treatment outcomes without requiring combination pharmacotherapy.
Combining phentermine with Wegovy is generally not recommended without specialist medical supervision, as there is no official UK guidance approving this combination and the safety profile has not been adequately studied in clinical trials. The combination may increase cardiovascular risks and amplify side effects.
Phentermine is not currently licensed in the UK and is only available via named-patient import. It is classified as a controlled substance due to its stimulant properties and is typically prescribed for short-term use only.
Before considering combination therapy, healthcare professionals recommend optimising the current medication dose, ensuring adequate treatment duration, and strengthening lifestyle interventions. Referral to a specialist tier 3 or 4 weight management service is appropriate for comprehensive assessment and consideration of alternative evidence-based approaches.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.