
Many individuals with hypothyroidism struggle with weight management and wonder whether Wegovy (semaglutide 2.4 mg) can be safely used alongside thyroid hormone replacement therapy. Wegovy is a GLP-1 receptor agonist licensed in the UK for weight management in adults with obesity or overweight with weight-related comorbidities. Current evidence suggests that Wegovy can generally be used safely in patients with well-controlled hypothyroidism on stable levothyroxine therapy. However, important safety considerations exist, particularly regarding thyroid C-cell tumours and the need for ongoing thyroid function monitoring. This article examines the safety, monitoring requirements, and practical considerations for using Wegovy in patients with hypothyroidism.
Quick Answer: Wegovy can generally be used safely in patients with well-controlled hypothyroidism on stable levothyroxine replacement therapy, though certain thyroid-related contraindications must be excluded.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereWegovy (semaglutide 2.4 mg) is a glucagon-like peptide-1 (GLP-1) receptor agonist licensed in the UK for weight management in adults with obesity or overweight with weight-related comorbidities. According to NICE Technology Appraisal 875, Wegovy is recommended for adults with a BMI of at least 35 kg/m² (or ≥30 kg/m² with weight-related comorbidities) and who are referred to specialist tier 3 weight management services. It works by mimicking the action of the naturally occurring hormone GLP-1, which regulates appetite and food intake. Wegovy slows gastric emptying, increases feelings of fullness, and reduces hunger signals in the brain, which can lead to reduced calorie intake and potential weight loss.
Hypothyroidism is a common endocrine disorder characterised by insufficient production of thyroid hormones (thyroxine, T4, and triiodothyronine, T3) by the thyroid gland. This condition affects metabolism, energy levels, and numerous bodily functions. In the UK, hypothyroidism affects approximately 2% of the population, with higher prevalence in women and older adults (NICE NG145). Most cases are managed with levothyroxine replacement therapy, which aims to restore normal thyroid hormone levels.
Many individuals with hypothyroidism struggle with weight management, as the condition can slow metabolic rate and contribute to weight gain. This has led to increased interest in whether weight management medications like Wegovy can be safely used alongside thyroid hormone replacement therapy. Understanding the relationship between these two medications is essential for patients and healthcare professionals when considering treatment options for weight management in people with thyroid disorders.
The intersection of obesity management and thyroid disease requires careful consideration of both the potential benefits of weight loss and any specific safety concerns related to combining these therapeutic approaches.
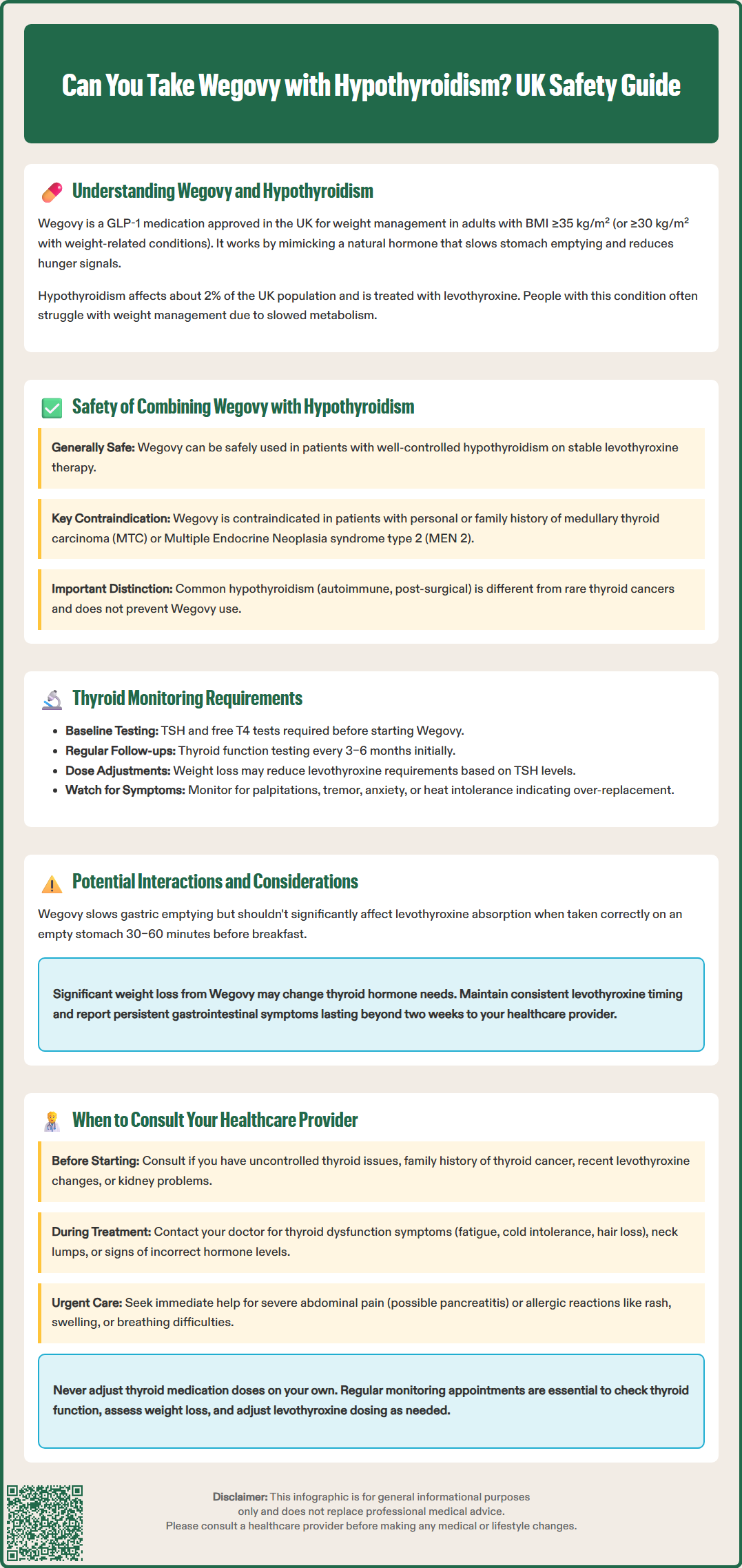
Current evidence suggests that Wegovy can generally be used safely in patients with well-controlled hypothyroidism who are on stable levothyroxine replacement therapy. According to the Wegovy Summary of Product Characteristics (SmPC), hypothyroidism itself is not listed as a contraindication to semaglutide use.
However, there is an important safety consideration regarding thyroid C-cell tumours. In rodent studies, GLP-1 receptor agonists, including semaglutide, have been associated with thyroid C-cell tumours (medullary thyroid carcinoma). Whilst the relevance of these findings to humans remains uncertain, Wegovy is contraindicated in patients with:
Personal or family history of medullary thyroid carcinoma (MTC)
Multiple endocrine neoplasia syndrome type 2 (MEN 2)
Other important contraindications for Wegovy include:
Pregnancy and breastfeeding
Severe renal impairment (eGFR <30 ml/min/1.73m²)
Hypersensitivity to semaglutide or any excipients
It is crucial to distinguish between these rare thyroid cancers and common hypothyroidism. Standard hypothyroidism (whether autoimmune thyroiditis, post-surgical, or other causes) does not represent a contraindication to Wegovy use. The thyroid C-cells that may be affected in animal studies are distinct from the follicular cells involved in typical hypothyroidism and thyroid hormone production.
Healthcare professionals should take a thorough medical history to exclude contraindications related to thyroid malignancy before prescribing Wegovy to any patient, including those with hypothyroidism.
Patients should report any suspected adverse reactions to Wegovy via the MHRA Yellow Card scheme (yellowcard.mhra.gov.uk).
Patients with pre-existing hypothyroidism require ongoing thyroid function monitoring, regardless of whether they are taking Wegovy. Standard practice involves checking thyroid-stimulating hormone (TSH) levels periodically to ensure levothyroxine dosing remains appropriate. NICE guidance (NG145) recommends annual thyroid function tests for patients on stable levothyroxine replacement, with more frequent monitoring if symptoms suggest under- or over-replacement.
When initiating Wegovy in patients with hypothyroidism, healthcare providers should consider:
Establishing baseline thyroid function (TSH and free T4) before starting treatment
Ensuring hypothyroidism is well-controlled with stable thyroid hormone levels
Planning follow-up thyroid function tests at regular intervals (typically 3–6 months initially)
Monitoring for symptoms of thyroid dysfunction during weight loss
Weight loss itself can potentially affect levothyroxine requirements. As body weight decreases, some patients may require adjustments to their thyroid hormone replacement dose. It's important to note that while weight is one factor in levothyroxine dosing, adjustments should be guided primarily by TSH levels rather than weight alone (BNF). Symptoms of over-replacement (such as palpitations, tremor, anxiety, or heat intolerance) should prompt thyroid function testing.
Gastrointestinal side effects from Wegovy—particularly nausea, vomiting, or diarrhoea—could theoretically affect levothyroxine absorption if severe. Patients should be advised to take levothyroxine consistently (typically on an empty stomach, 30–60 minutes before breakfast) and report any persistent gastrointestinal symptoms to their healthcare provider. If severe gastrointestinal symptoms persist for more than two weeks, earlier thyroid function testing may be warranted. Regular monitoring ensures any necessary dose adjustments can be made promptly to maintain optimal thyroid function throughout weight management treatment.
Pharmacokinetic interactions between Wegovy and levothyroxine require consideration. The Wegovy SmPC notes that semaglutide delays gastric emptying, which could potentially impact the absorption of concomitant oral medications. While there is no specific interaction listed with levothyroxine, several practical considerations merit attention when using these medications together.
Gastric emptying effects: Semaglutide significantly slows gastric emptying as part of its mechanism of action. Whilst this primarily affects the absorption of orally administered medications taken with food, levothyroxine is typically taken on an empty stomach, which should minimise any absorption interference. Nevertheless, patients experiencing significant gastrointestinal side effects should maintain consistent timing of levothyroxine administration and report any concerns to their healthcare provider.
Weight loss and metabolic changes: Substantial weight reduction achieved with Wegovy can alter metabolic parameters and may influence thyroid hormone requirements. Some patients may need levothyroxine dose adjustments as they lose weight, based on TSH monitoring results.
Cardiovascular considerations: Both hypothyroidism and obesity are risk factors for cardiovascular disease. Patients with hypothyroidism may have underlying cardiac conditions that require consideration when initiating weight management therapy. While semaglutide 1 mg (Ozempic) has demonstrated cardiovascular benefits in clinical trials, the cardiovascular outcomes data for the higher 2.4 mg dose used in Wegovy is still under investigation. Individual patient assessment remains essential.
Other safety considerations: Patients should be aware of other important Wegovy safety warnings, including risks of pancreatitis, gallbladder disease, and diabetic retinopathy complications in those with diabetes.
Other medications: Patients with hypothyroidism often take multiple medications. Healthcare providers should review the complete medication list for potential interactions with Wegovy, including drugs affecting gastrointestinal motility, glucose metabolism, or cardiovascular function. Particular attention should be paid to diabetes medications, as Wegovy significantly affects glycaemic control and may necessitate dose adjustments of concurrent antidiabetic agents.
Patients with hypothyroidism considering Wegovy should have a thorough discussion with their healthcare provider before starting treatment. In the UK, Wegovy is prescribed via specialist tier 3 weight management services according to NICE TA875. This consultation should cover personal and family thyroid history, current thyroid function status, and overall suitability for weight management medication.
Seek medical advice before starting Wegovy if you have:
Uncontrolled or recently diagnosed hypothyroidism
Personal or family history of thyroid cancer (particularly medullary thyroid carcinoma)
Multiple endocrine neoplasia syndrome type 2
Recent changes to levothyroxine dosing
Symptoms suggesting inadequate thyroid hormone replacement
Pregnancy, planning pregnancy, or breastfeeding
Severe kidney problems
Contact your healthcare provider during Wegovy treatment if you experience:
Symptoms of thyroid dysfunction: fatigue, cold intolerance, weight changes beyond expected, hair loss, or palpitations
Neck symptoms: persistent lump, swelling, pain, or difficulty swallowing
Severe gastrointestinal side effects: persistent vomiting or diarrhoea that might affect medication absorption
Signs of over- or under-replacement: anxiety, tremor, heat intolerance (over-replacement) or lethargy, constipation, depression (under-replacement)
Seek urgent medical attention if you develop:
Severe, persistent abdominal pain (possible pancreatitis)
Signs of allergic reaction (rash, swelling, difficulty breathing)
Regular follow-up appointments are essential for patients with hypothyroidism using Wegovy. These should include thyroid function monitoring, assessment of weight loss progress, evaluation of side effects, and review of levothyroxine dosing requirements. Your healthcare provider may recommend more frequent monitoring initially, particularly during the dose escalation phase of Wegovy.
If you develop any concerning symptoms or have questions about your treatment, do not hesitate to contact your healthcare provider. Never adjust thyroid medication doses without medical supervision, as both under- and over-replacement can have significant health consequences. Collaborative care between patients and healthcare providers ensures safe, effective use of Wegovy in individuals with hypothyroidism.
Report any suspected side effects to the MHRA Yellow Card scheme at yellowcard.mhra.gov.uk.
No, standard hypothyroidism is not a contraindication to Wegovy. However, personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 are absolute contraindications to semaglutide use.
Patients with hypothyroidism should continue regular thyroid function monitoring, typically every 3–6 months initially when starting Wegovy, as weight loss may affect levothyroxine requirements. Adjustments should be guided by TSH levels rather than weight alone.
Wegovy delays gastric emptying, which could theoretically affect absorption of oral medications. However, levothyroxine is typically taken on an empty stomach, minimising potential interference. Patients should maintain consistent timing of levothyroxine administration and report persistent gastrointestinal symptoms.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.