
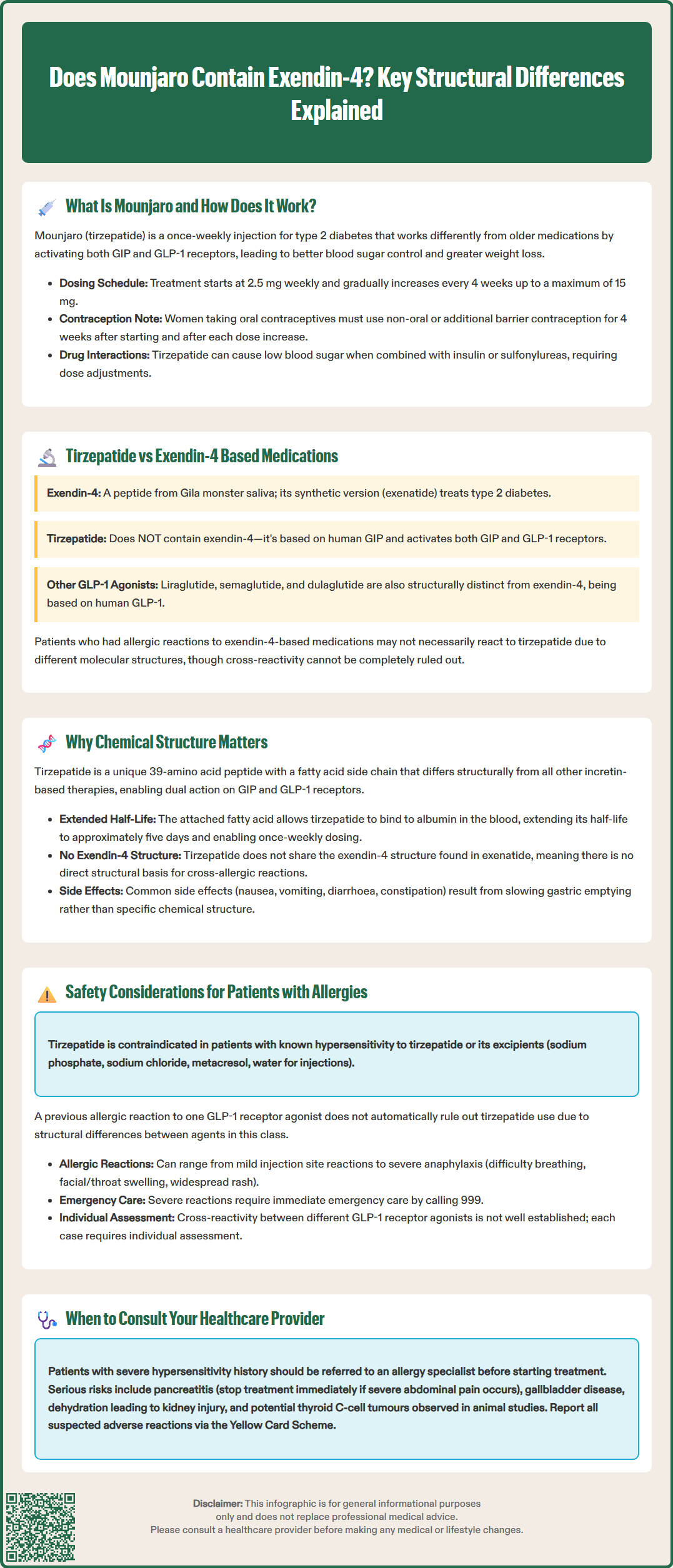
Mounjaro (tirzepatide) is a dual GIP and GLP-1 receptor agonist used for type 2 diabetes and weight management in the UK. A common question amongst patients and prescribers is whether Mounjaro contains exendin-4, a peptide found in earlier GLP-1 medications like exenatide. Understanding the structural composition of tirzepatide is particularly important for individuals with medication allergies or previous adverse reactions to incretin-based therapies. This article clarifies the chemical differences between tirzepatide and exendin-4, explains why these distinctions matter clinically, and provides guidance on safety considerations for patients with GLP-1 medication sensitivities.
Quick Answer: Mounjaro (tirzepatide) does not contain exendin-4 and is not structurally derived from it.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereMounjaro (tirzepatide) is a prescription medicine authorised in the UK for the treatment of type 2 diabetes mellitus. Tirzepatide is also available under the brand name Zepbound for chronic weight management in adults with obesity or overweight with weight-related comorbidities. It is administered as a once-weekly subcutaneous injection and represents a novel class of medication known as a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist.
Unlike earlier GLP-1 receptor agonists, tirzepatide activates both GIP and GLP-1 receptors simultaneously. This dual mechanism enhances insulin secretion in a glucose-dependent manner, suppresses glucagon release when blood glucose is elevated, slows gastric emptying, and reduces appetite through central nervous system pathways. The combined action on both incretin pathways results in significant improvements in glycaemic control and substantial weight loss, often exceeding that seen with GLP-1 receptor agonists alone.
Clinical trials, including the SURPASS programme for diabetes and the SURMOUNT studies for weight management, have demonstrated tirzepatide's efficacy in reducing HbA1c levels and body weight. Treatment typically starts with a 2.5 mg weekly dose for 4 weeks (for initiation only, not intended for glycaemic control), followed by stepwise increases every 4 weeks (to 5 mg, 7.5 mg, 10 mg, 12.5 mg, and 15 mg) as tolerated. This gradual titration helps minimise gastrointestinal side effects, which are the most commonly reported adverse reactions.
Important safety considerations include the risk of hypoglycaemia when used with insulin or sulfonylureas, potential interactions with oral contraceptives (requiring non-oral contraception or additional barrier methods for 4 weeks after initiation and each dose increase), risk of pancreatitis, gallbladder disease, and dehydration potentially leading to acute kidney injury. Tirzepatide is not indicated for type 1 diabetes or diabetic ketoacidosis.
Understanding the pharmacological basis of tirzepatide is essential for both prescribers and patients, particularly when considering its structural differences from other incretin-based therapies and the implications for those with medication allergies.
Exendin-4 is a naturally occurring peptide originally isolated from the saliva of the Gila monster lizard (Heloderma suspectum). It shares approximately 53% amino acid sequence homology with human GLP-1 and acts as a potent GLP-1 receptor agonist. Exenatide (marketed as Byetta and Bydureon in the UK) is a synthetic version of exendin-4 and was one of the first GLP-1 receptor agonists approved for clinical use in type 2 diabetes.
Tirzepatide, the active ingredient in Mounjaro and Zepbound, does not contain exendin-4 and is not structurally derived from it. Instead, tirzepatide is a synthetic peptide based on the human GIP sequence, modified to include a C20 fatty diacid chain that enables albumin binding and prolongs its half-life. This structural modification allows for once-weekly dosing. Importantly, tirzepatide has been engineered to activate both GIP and GLP-1 receptors, whereas exendin-4 and exenatide act solely on GLP-1 receptors.
The amino acid sequence of tirzepatide differs substantially from exendin-4. While both medications influence incretin pathways, their molecular structures, receptor binding profiles, and pharmacokinetic properties are distinct. This is clinically relevant because patients who have experienced allergic reactions or adverse effects to exendin-4-based medications (such as exenatide) may not necessarily react to tirzepatide, though cross-reactivity cannot be entirely excluded without formal assessment. The evidence on cross-reactivity between different GLP-1 receptor agonists is limited, and individualised clinical assessment is recommended.
Other GLP-1 receptor agonists available in the UK, such as liraglutide (Victoza, Saxenda), semaglutide (Ozempic, Wegovy), and dulaglutide (Trulicity), are also structurally different from exendin-4. These are analogues of human GLP-1 rather than exendin-4 derivatives, further highlighting the diversity within this therapeutic class.
The chemical structure of a medication fundamentally determines its pharmacological activity, safety profile, and potential for adverse reactions, including allergic responses. Tirzepatide's unique structure—a 39-amino acid peptide with a fatty acid side chain—distinguishes it from all other incretin-based therapies currently available.
From a mechanistic perspective, tirzepatide's dual agonism at GIP and GLP-1 receptors is made possible by specific amino acid substitutions and modifications that allow it to bind effectively to both receptor types. The addition of the C20 fatty diacid moiety facilitates binding to albumin in the bloodstream, which prolongs the drug's half-life to approximately five days and permits once-weekly administration, as documented in the product's Summary of Product Characteristics (SmPC).
For patients and prescribers, understanding these structural differences is important when considering medication allergies or hypersensitivity reactions. Allergic reactions to peptide-based drugs can be directed against the peptide backbone itself, excipients in the formulation, or degradation products. Because tirzepatide does not share the exendin-4 structure, there is no direct structural basis for cross-reactivity with exenatide. However, patients with a history of reactions to any GLP-1 receptor agonist should be carefully assessed, as class-related effects (such as gastrointestinal intolerance) may still occur.
Additionally, the structural properties of tirzepatide influence its side effect profile. Common adverse effects include nausea, vomiting, diarrhoea, and constipation, which are related to the drug's mechanism of slowing gastric emptying rather than its specific chemical structure. Rare but serious risks include pancreatitis (patients should be advised to stop treatment and seek urgent medical attention if they experience severe, persistent abdominal pain, with or without vomiting), gallbladder disease, and dehydration potentially leading to acute kidney injury. Thyroid C-cell tumours have been observed in rodent studies with GLP-1 receptor agonists including tirzepatide, though the human relevance of this finding is uncertain.
Patients with a documented allergy or hypersensitivity to GLP-1 receptor agonists require careful evaluation before starting Mounjaro. Tirzepatide is contraindicated in individuals with a known hypersensitivity to tirzepatide or any of the excipients in the formulation (including sodium phosphate, sodium chloride, metacresol, and water for injections). However, a history of reaction to one GLP-1 receptor agonist does not automatically preclude the use of tirzepatide, given the structural differences between agents in this class.
Allergic reactions to GLP-1 receptor agonists, though uncommon, can range from mild injection site reactions to severe systemic hypersensitivity, including anaphylaxis. Symptoms may include rash, pruritus, urticaria, angioedema, or respiratory distress. If a patient has experienced a confirmed allergic reaction to exenatide (an exendin-4 derivative), switching to tirzepatide may be considered, as there is no structural overlap. Nonetheless, this decision should be made collaboratively with the patient's GP or specialist, ideally with input from an allergist or immunologist if the prior reaction was severe.
Cross-reactivity between different GLP-1 receptor agonists is not well characterised in the literature, and there is no official guidance suggesting automatic cross-sensitivity. Each case must be assessed individually, considering the nature and severity of the previous reaction, the patient's clinical need for the medication, and the availability of alternative therapies.
Patients starting Mounjaro should be counselled on recognising signs of allergic reactions and advised to seek urgent medical attention or call 999 if they develop symptoms of anaphylaxis such as difficulty breathing, swelling of the face or throat, or widespread rash. Injection site reactions—such as redness, itching, or swelling—are generally mild and self-limiting but should be reported if persistent or worsening.
For individuals with a history of severe hypersensitivity reactions, referral to an allergy or immunology specialist may be appropriate before initiating treatment. Additionally, patients should be reminded that gastrointestinal side effects, while common, are not allergic in nature and typically improve with continued use or dose adjustment. Clear communication between patients and healthcare providers is essential to ensure safe and effective use of Mounjaro.
Patients and healthcare professionals are encouraged to report suspected adverse reactions to tirzepatide via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store.
Tirzepatide (Mounjaro) does not contain exendin-4 and is structurally different from exenatide, so there is no direct basis for cross-reactivity. However, any history of allergic reactions to GLP-1 receptor agonists should be discussed with your GP or specialist before starting Mounjaro.
Tirzepatide is a synthetic peptide based on human GIP that activates both GIP and GLP-1 receptors, whilst exendin-4 is a naturally occurring peptide that acts only on GLP-1 receptors. Their amino acid sequences and molecular structures are distinct.
If you develop symptoms of anaphylaxis such as difficulty breathing, facial or throat swelling, or widespread rash, call 999 immediately. For milder reactions such as persistent injection site reactions, contact your GP or healthcare provider and report the reaction via the MHRA Yellow Card Scheme.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.