
Does Rybelsus increase blood pressure? This is a common concern for patients prescribed this type 2 diabetes medication. Rybelsus (semaglutide) is a glucagon-like peptide-1 (GLP-1) receptor agonist licensed in the UK for glycaemic control. Clinical evidence indicates that Rybelsus does not typically raise blood pressure; in fact, most patients experience stable readings or modest reductions in both systolic and diastolic blood pressure during treatment. This article examines the relationship between Rybelsus and blood pressure, reviews clinical trial data, and provides guidance on monitoring cardiovascular parameters whilst taking this medication.
Quick Answer: Rybelsus (semaglutide) does not typically increase blood pressure and may produce modest reductions in systolic and diastolic readings in many patients with type 2 diabetes.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereRybelsus (semaglutide) does not typically increase blood pressure. In fact, clinical evidence suggests that this medication may have a modest blood pressure-lowering effect in many patients. Rybelsus is a glucagon-like peptide-1 (GLP-1) receptor agonist licensed in the UK for the treatment of type 2 diabetes mellitus. It works by mimicking the action of the naturally occurring hormone GLP-1, which stimulates insulin secretion, suppresses glucagon release, and slows gastric emptying.
The relationship between Rybelsus and blood pressure is generally favourable, with most patients experiencing either stable readings or slight reductions in both systolic and diastolic blood pressure during treatment. This effect is thought to be related to weight loss and improved glycaemic control, though other mechanisms may contribute. According to the UK Summary of Product Characteristics (SmPC), hypertension is not listed as an adverse reaction for Rybelsus. It is worth noting, however, that GLP-1 receptor agonists including semaglutide are associated with small mean increases in heart rate (approximately 2-3 beats per minute).
Individual responses to medication can vary considerably. Whilst the majority of patients do not experience blood pressure increases, it remains important to monitor cardiovascular parameters regularly when starting or adjusting diabetes medications. Patients with pre-existing hypertension should continue their prescribed antihypertensive medications unless advised otherwise by their healthcare provider. Any concerns about blood pressure changes during Rybelsus treatment should be discussed with a GP or diabetes specialist, who can assess whether adjustments to monitoring or treatment are necessary.
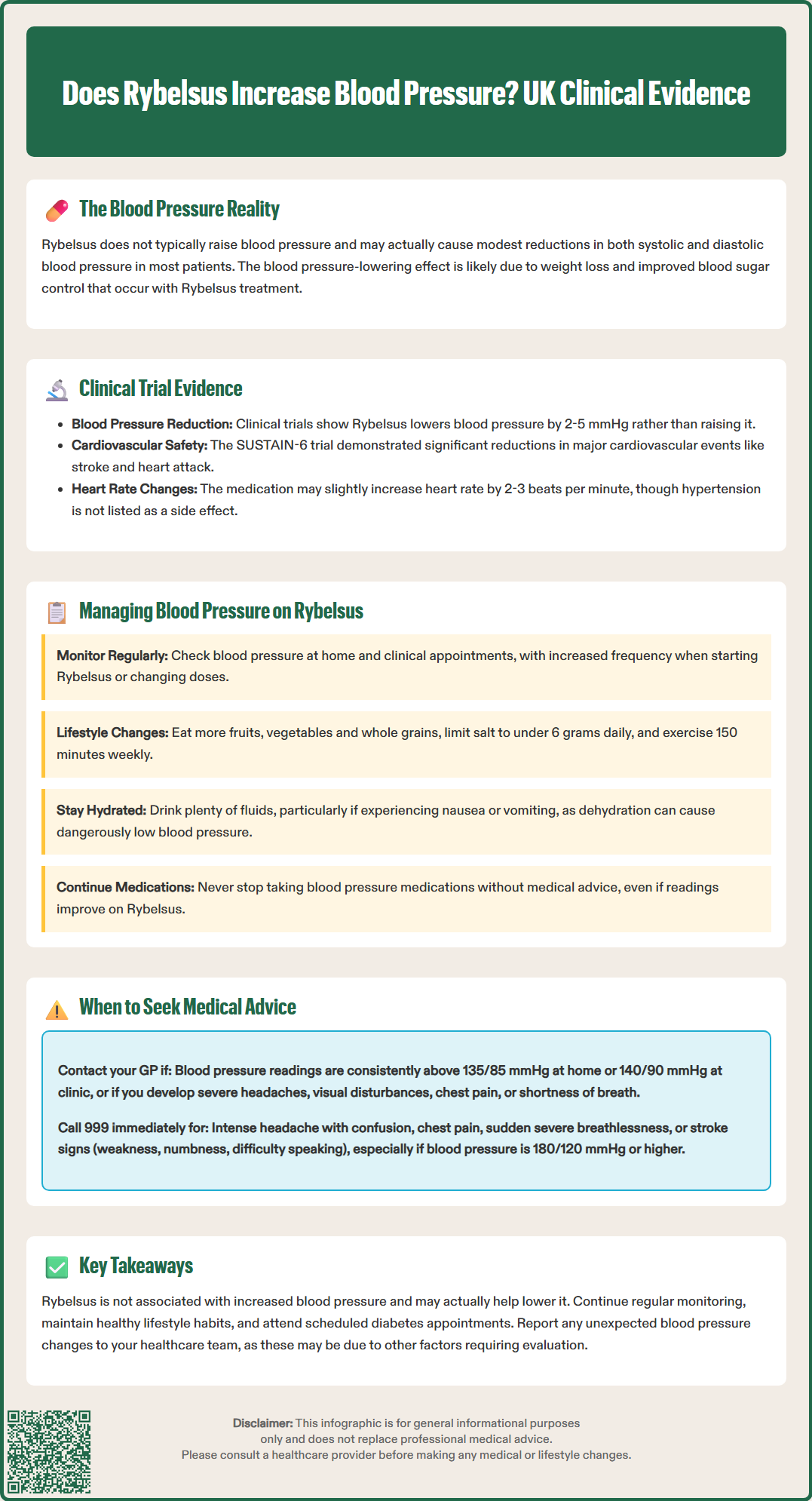
Clinical trial data demonstrates that semaglutide, the active ingredient in Rybelsus, is associated with modest reductions in blood pressure rather than increases. The PIONEER clinical trial programme, which evaluated oral semaglutide across diverse patient populations with type 2 diabetes, showed small but statistically significant decreases in systolic blood pressure. In the PIONEER 1 trial, patients receiving semaglutide 14 mg (the maximum licensed dose) experienced mean reductions in systolic blood pressure of approximately 2–5 mmHg compared to placebo.
These blood pressure reductions appear to be partly related to weight loss, though other factors may contribute to the overall cardiovascular effect. The precise mechanisms behind this blood pressure-lowering effect are not fully established but have been hypothesised to involve changes in endothelial function, fluid balance, and vascular tone.
The cardiovascular outcomes trial SUSTAIN-6, which studied injectable semaglutide (a related formulation), demonstrated significant reductions in major adverse cardiovascular events, including non-fatal stroke and non-fatal myocardial infarction. Whilst this trial used the subcutaneous formulation rather than oral Rybelsus, the findings support the cardiovascular safety profile of semaglutide. The National Institute for Health and Care Excellence (NICE) guideline NG28 for type 2 diabetes management recognises the evidence for cardiovascular risk reduction with certain GLP-1 receptor agonists when used appropriately.
It is worth noting that no signal of clinically significant blood pressure elevation has been identified in major trials or the UK SmPC for Rybelsus. Patients who experience unexpected blood pressure changes should have alternative causes investigated, including medication interactions, dietary factors, or progression of underlying cardiovascular disease.
Effective blood pressure management during Rybelsus treatment requires a comprehensive approach that addresses lifestyle factors, monitors cardiovascular parameters, and optimises concurrent medications. Patients should continue regular blood pressure monitoring as recommended by their healthcare team, typically at home and during routine clinical appointments. The frequency of monitoring may be increased when initiating Rybelsus or adjusting the dose, particularly in patients with pre-existing hypertension or cardiovascular disease.
Lifestyle modifications remain fundamental to blood pressure control and complement the effects of Rybelsus. These include:
Dietary adjustments: Following a balanced diet rich in fruits, vegetables, and whole grains whilst limiting salt intake to less than 6 grams per day (approximately 2.4g sodium) in line with NHS recommendations
Regular physical activity: Aiming for at least 150 minutes of moderate-intensity aerobic exercise weekly, as recommended by NHS physical activity guidelines
Weight management: Rybelsus often facilitates weight loss, which independently improves blood pressure control
Alcohol moderation: Limiting intake to within UK Chief Medical Officers' guidelines (14 units per week spread over several days)
Smoking cessation: Accessing NHS Stop Smoking Services if applicable
Medication optimisation is equally important. Patients taking antihypertensive medications should not discontinue these without medical advice, even if blood pressure readings improve on Rybelsus. However, healthcare providers may need to adjust antihypertensive doses if blood pressure falls significantly, particularly to prevent hypotension (excessively low blood pressure). Common symptoms of hypotension include dizziness, light-headedness, and fatigue, especially upon standing.
It's important to maintain adequate hydration, particularly if experiencing gastrointestinal side effects of Rybelsus such as nausea, vomiting or diarrhoea, which could contribute to dehydration and hypotension. Home blood pressure monitoring, when performed correctly using a validated device (such as those listed by the British and Irish Hypertension Society), provides valuable information for clinical decision-making. Measurements should be taken after 5 minutes of rest, in a seated position with an appropriately sized cuff, and recorded systematically for review at appointments.
Patients should contact their GP or diabetes care team promptly if they experience significant changes in blood pressure readings or related symptoms whilst taking Rybelsus. Specific situations warranting medical review include persistently elevated blood pressure readings (consistently above 135/85 mmHg on home monitoring or above 140/90 mmHg in clinic measurements, in line with NICE guideline NG136), or new symptoms such as severe headaches, visual disturbances, chest pain, or shortness of breath.
Symptoms potentially indicating blood pressure problems include:
Persistent or severe headaches
Visual changes, including blurred vision or seeing spots
Chest discomfort or palpitations
Unexplained dizziness or fainting episodes
Severe fatigue or confusion
Immediate medical attention (999 or A&E) is required if patients experience symptoms suggesting a hypertensive emergency, such as severe headache with confusion, chest pain suggestive of myocardial infarction, sudden severe shortness of breath, or neurological symptoms including weakness, numbness, or difficulty speaking that could indicate stroke. Blood pressure readings of 180/120 mmHg or higher with symptoms of acute target organ damage warrant same-day medical assessment.
Conversely, symptoms of hypotension (excessively low blood pressure) also warrant medical review. These include persistent dizziness upon standing (postural hypotension), fainting episodes, unusual fatigue, or blurred vision. Patients taking multiple antihypertensive medications alongside Rybelsus may be at higher risk and should have their treatment regimen reviewed if such symptoms develop.
Routine monitoring remains essential. Even in the absence of symptoms, patients should attend scheduled diabetes review appointments where blood pressure is routinely assessed. NICE guidelines recommend at least annual blood pressure checks for people with diabetes, though more frequent monitoring may be appropriate depending on individual cardiovascular risk profiles. For urgent but non-emergency concerns, NHS 111 can provide advice. Patients who suspect they are experiencing side effects from Rybelsus should report these through the MHRA Yellow Card scheme (yellowcard.mhra.gov.uk or via the Yellow Card app).
No, Rybelsus does not typically cause high blood pressure. Clinical trial data shows that semaglutide is associated with modest reductions in systolic blood pressure of approximately 2–5 mmHg, and hypertension is not listed as an adverse reaction in the UK Summary of Product Characteristics.
No, you should not stop taking antihypertensive medications without medical advice. Your healthcare provider may adjust doses if your blood pressure falls significantly during Rybelsus treatment, but continue all prescribed medications unless specifically instructed otherwise.
Blood pressure monitoring frequency depends on your individual cardiovascular risk profile. NICE guidelines recommend at least annual checks for people with diabetes, though more frequent monitoring may be appropriate when initiating Rybelsus or if you have pre-existing hypertension.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.