LOSE WEIGHT WITH MEDICAL SUPPORT — BUILT FOR MEN
- Your personalised programme is built around medical care, not willpower.
- No generic diets. No guesswork.
- Just science-backed results and expert support.
Find out if you’re eligible

Mounjaro (tirzepatide) is a dual GIP and GLP-1 receptor agonist licensed in the UK for type 2 diabetes and weight management. Many patients with autoimmune conditions also develop metabolic complications, raising important questions about whether Mounjaro is safe and appropriate for this population. Whilst tirzepatide is not contraindicated in autoimmune disease, individual assessment is essential. This article examines the evidence, safety considerations, and practical guidance for patients with autoimmune conditions considering Mounjaro, in line with UK regulatory guidance from the MHRA and clinical recommendations from NICE.
Quick Answer: Mounjaro (tirzepatide) is not contraindicated in autoimmune disease, but individual assessment by a healthcare professional is essential before starting treatment.
Mounjaro (tirzepatide) is a prescription medicine licensed in the UK for the treatment of type 2 diabetes mellitus and, more recently, for weight management in adults with obesity or overweight with weight-related comorbidities, as per the Medicines and Healthcare products Regulatory Agency (MHRA) and European Medicines Agency (EMA) authorisations. It represents a novel class of medication known as a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist.
The mechanism of action involves mimicking two naturally occurring incretin hormones that play crucial roles in glucose regulation and appetite control. When blood glucose levels rise after eating, tirzepatide stimulates insulin secretion from pancreatic beta cells whilst simultaneously suppressing glucagon release from alpha cells. This dual action helps to lower blood glucose levels in a glucose-dependent manner, meaning the risk of hypoglycaemia is reduced when glucose levels are normal. However, the risk of hypoglycaemia increases when tirzepatide is used in combination with insulin or sulfonylureas. Additionally, tirzepatide slows gastric emptying and acts on appetite centres in the brain to promote satiety, leading to reduced caloric intake and subsequent weight loss.
Tirzepatide is administered once weekly via subcutaneous injection, typically in the abdomen, thigh, or upper arm. Treatment usually starts at 2.5 mg once weekly, with stepwise increases to reach the maintenance dose. The dosing is gradually escalated over several weeks to minimise gastrointestinal side effects. Important safety considerations include warnings for pancreatitis, gallbladder disease, diabetic retinopathy, and severe gastrointestinal disease (including severe gastroparesis), for which tirzepatide is not recommended.
Autoimmune diseases encompass a diverse group of conditions in which the immune system mistakenly attacks the body's own tissues, causing inflammation and damage to various organs and systems. Common autoimmune conditions include rheumatoid arthritis, systemic lupus erythematosus (SLE), inflammatory bowel disease (Crohn's disease and ulcerative colitis), psoriasis, multiple sclerosis, type 1 diabetes, and autoimmune thyroid disorders such as Hashimoto's thyroiditis and Graves' disease.
The pathophysiology of autoimmune diseases involves a breakdown in immune tolerance, where self-antigens are recognised as foreign. This triggers an inappropriate immune response involving T cells, B cells, and the production of autoantibodies. The resulting chronic inflammation can lead to tissue destruction, organ dysfunction, and systemic symptoms including fatigue, pain, and reduced quality of life. Many autoimmune conditions follow a relapsing-remitting course, with periods of active disease (flares) alternating with remission.
Management of autoimmune diseases often requires immunosuppressive or immunomodulatory therapies, including corticosteroids, disease-modifying antirheumatic drugs (DMARDs), biologics, and targeted synthetic agents. These treatments aim to reduce inflammation, prevent disease progression, and maintain remission. When considering additional medications such as Mounjaro for comorbid conditions like type 2 diabetes or obesity, clinicians must carefully evaluate potential interactions with the immune system and existing immunosuppressive regimens. The complexity of autoimmune disease management necessitates individualised treatment approaches and close monitoring for both disease activity and medication-related adverse effects.
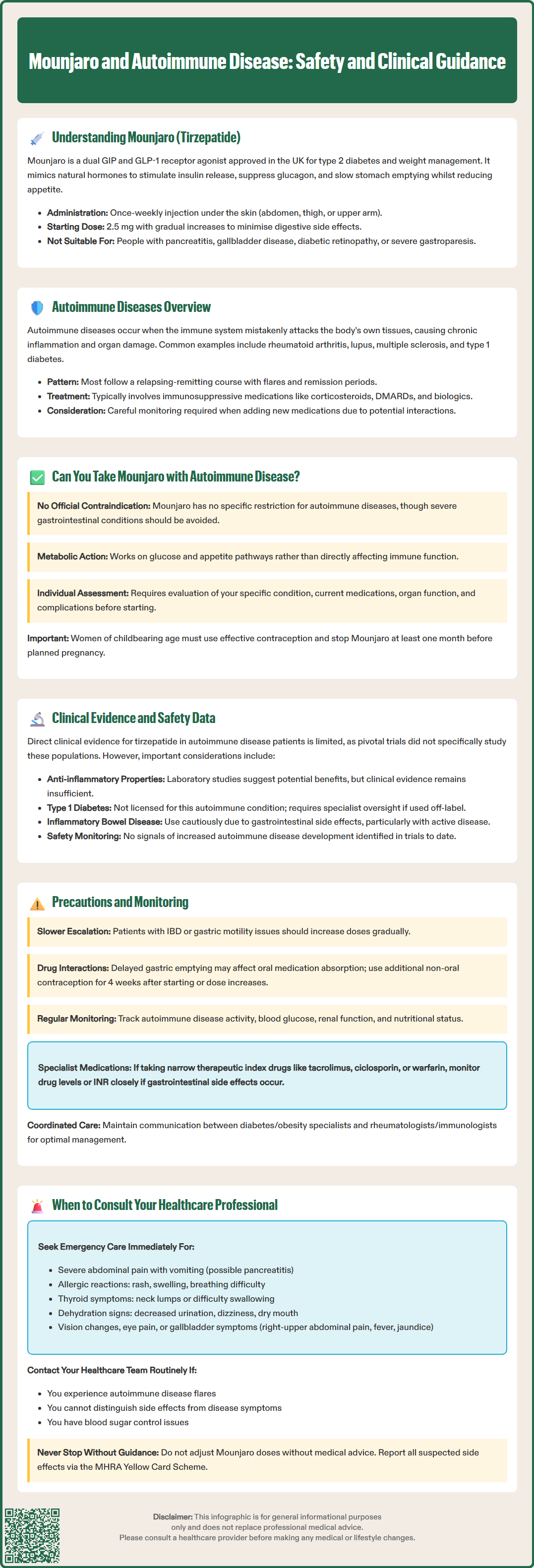
The question of whether individuals with autoimmune diseases can safely take Mounjaro is clinically relevant, as many people with autoimmune conditions also develop metabolic complications such as type 2 diabetes or obesity. There is no official contraindication to using tirzepatide in patients with autoimmune diseases listed in the Summary of Product Characteristics (SmPC) approved by the MHRA and EMA. However, it is important to note that tirzepatide is not recommended in severe gastrointestinal disease, including severe gastroparesis, which may be present in some autoimmune conditions.
Tirzepatide's mechanism of action primarily targets metabolic pathways related to glucose homeostasis and appetite regulation rather than directly modulating immune function. Unlike immunosuppressive medications, GLP-1 and GIP receptor agonists do not intentionally suppress or alter immune responses. However, it is important to note that the clinical trial populations for Mounjaro predominantly included patients with type 2 diabetes and obesity, and whilst some participants likely had concurrent autoimmune conditions, these were not specifically studied as separate subgroups in most trials.
Individual assessment is crucial when considering Mounjaro for patients with autoimmune disease. Factors to consider include:
The specific autoimmune condition and its current activity status
Concurrent immunosuppressive or immunomodulatory medications
Overall disease burden and organ function
Presence of complications such as gastroparesis (particularly relevant in autoimmune conditions affecting the gastrointestinal tract)
Treatment goals and potential benefits versus risks
Patients with well-controlled autoimmune disease who require treatment for type 2 diabetes or weight management may be suitable candidates for Mounjaro, provided there are no other contraindications. It is important to note that tirzepatide is not licensed for type 1 diabetes. Women of childbearing potential should use effective contraception while taking tirzepatide, and the medication should be discontinued at least 1 month before a planned pregnancy. Tirzepatide is not recommended during pregnancy or breastfeeding. The decision should be made collaboratively between the patient and their healthcare team, considering the full clinical picture and in accordance with NICE guidance.
Direct clinical evidence specifically examining tirzepatide use in patients with autoimmune diseases remains limited. The pivotal SURPASS clinical trial programme, which established Mounjaro's efficacy and safety profile, did not specifically focus on autoimmune disease subpopulations. However, post-marketing surveillance and real-world data are gradually accumulating as the medication becomes more widely prescribed.
Some theoretical considerations warrant attention. Emerging preclinical research suggests that GLP-1 receptor agonists may have anti-inflammatory properties beyond their metabolic effects. Some laboratory studies have demonstrated that GLP-1 receptor activation can reduce inflammatory cytokine production and oxidative stress. Whilst this could theoretically be beneficial in autoimmune conditions characterised by chronic inflammation, there is insufficient clinical evidence to confirm meaningful immunomodulatory effects in humans at therapeutic doses, and these observations remain largely speculative at present.
Regarding specific autoimmune conditions, some studies have investigated GLP-1 receptor agonists as adjunctive therapy to insulin in type 1 diabetes (an autoimmune disease). It is important to emphasise that tirzepatide is not licensed for type 1 diabetes, and use in this population would be off-label. If such off-label use were considered, specialist oversight would be essential, with careful monitoring for diabetic ketoacidosis risk, particularly if insulin doses are reduced. For inflammatory bowel disease, the gastrointestinal side effects of tirzepatide (nausea, vomiting, diarrhoea) may be particularly relevant, and caution is advised in patients with active disease or strictures.
Safety monitoring in clinical trials has not identified specific signals suggesting increased autoimmune disease development or exacerbation with tirzepatide. The overall adverse event profile appears consistent across patient populations. Important safety considerations from the SmPC include monitoring for gallbladder disease and diabetic retinopathy complications. Patients should be advised to report suspected side effects via the MHRA Yellow Card Scheme (www.mhra.gov.uk/yellowcard or the Yellow Card app). The absence of evidence is not evidence of absence, and ongoing pharmacovigilance continues to monitor for unexpected safety signals in diverse patient populations.
Patients with autoimmune diseases considering Mounjaro require individualised risk assessment and enhanced monitoring protocols. Several practical precautions should be implemented to ensure safe and effective treatment.
Gastrointestinal considerations are particularly important. Tirzepatide commonly causes nausea, vomiting, diarrhoea, and constipation, especially during dose escalation. For patients with inflammatory bowel disease, these side effects may be difficult to distinguish from disease flares. Similarly, individuals with autoimmune conditions affecting gastric motility (such as systemic sclerosis) may experience exacerbated symptoms due to tirzepatide's effect on gastric emptying. A slower dose titration schedule may be appropriate in these populations, and tirzepatide is not recommended in severe gastrointestinal disease, including severe gastroparesis.
Drug interactions warrant careful review. Many patients with autoimmune diseases take immunosuppressive medications, corticosteroids, or non-steroidal anti-inflammatory drugs (NSAIDs). Whilst tirzepatide does not have major pharmacokinetic interactions with these agents, the delayed gastric emptying it causes may affect the absorption of oral medications. Of particular importance, tirzepatide can reduce the exposure to oral contraceptives after initiation and after each dose increase; additional or non-oral contraception is advised for 4 weeks in these situations. Tirzepatide should not be combined with other GLP-1 receptor agonists, and there is limited value in combining it with DPP-4 inhibitors.
Monitoring parameters should include:
Regular assessment of autoimmune disease activity using validated measures
Blood glucose monitoring, particularly if on insulin or sulfonylureas (increased hypoglycaemia risk)
Renal function tests, especially during significant gastrointestinal adverse effects or dehydration
Nutritional status and weight trends
Gastrointestinal symptoms and their impact on quality of life
For patients on medicines with a narrow therapeutic index (e.g., tacrolimus, ciclosporin, warfarin), consider monitoring levels/INR if gastrointestinal adverse effects occur
Dose adjustments may be necessary based on tolerability and response. No dose adjustment is required for renal or hepatic impairment, but monitoring is advised if dehydration occurs. Patients should maintain regular contact with both their diabetes/obesity specialist and their rheumatologist or immunologist to ensure coordinated care. Any new or worsening symptoms should be promptly reported and evaluated to distinguish between medication side effects and autoimmune disease progression.
Patients with autoimmune diseases taking Mounjaro should maintain proactive communication with their healthcare team and be aware of specific situations requiring medical attention. Understanding when to seek advice ensures timely intervention and optimal safety.
Urgent consultation is warranted if you experience:
Severe abdominal pain, particularly if persistent or accompanied by vomiting, which could indicate pancreatitis (a rare but serious side effect)
Signs of allergic reaction, including rash, itching, swelling, severe dizziness, or difficulty breathing – call 999 or go to A&E immediately if you experience severe allergic reaction symptoms
Symptoms of thyroid problems, such as a lump or swelling in the neck, hoarseness, or difficulty swallowing (tirzepatide carries a warning regarding thyroid C-cell tumours based on animal studies)
Severe or persistent gastrointestinal symptoms that interfere with eating, drinking, or taking other essential medications
Signs of dehydration, including decreased urination, dizziness, or dry mouth, particularly if experiencing vomiting or diarrhoea
Changes in vision or eye pain, especially relevant for patients with diabetes
Right-upper-quadrant pain, fever, or yellowing of the skin/eyes, which could indicate gallbladder problems
For urgent but non-emergency concerns, contact NHS 111 for advice.
Routine consultation should occur if you notice:
New symptoms that could represent an autoimmune disease flare
Difficulty distinguishing between medication side effects and disease activity
Inadequate glycaemic control or unexpected blood glucose patterns
Significant weight loss or nutritional concerns
Problems with medication adherence or injection technique
Questions about interactions with new medications prescribed for your autoimmune condition
According to NICE guidance on diabetes management, regular review appointments should assess treatment effectiveness, side effects, and overall disease control. For patients with complex medical histories including autoimmune disease, multidisciplinary team involvement is often beneficial. Your GP, diabetes specialist nurse, and consultant should work collaboratively to optimise your treatment plan. Never discontinue Mounjaro or adjust your dose without medical guidance, and always inform all healthcare professionals about your complete medication list and medical history to ensure safe, coordinated care.
If you suspect you are experiencing side effects from Mounjaro, report them via the MHRA Yellow Card Scheme (www.mhra.gov.uk/yellowcard or the Yellow Card app).
Mounjaro is not contraindicated in rheumatoid arthritis or lupus, but individual assessment is required. Patients with well-controlled autoimmune disease may be suitable candidates, provided there are no other contraindications and their healthcare team agrees treatment is appropriate.
Tirzepatide does not have major pharmacokinetic interactions with immunosuppressive drugs, but its effect on gastric emptying may affect absorption of oral medications. Patients taking medicines with a narrow therapeutic index should have levels monitored if gastrointestinal side effects occur.
Mounjaro is not recommended in severe gastrointestinal disease. For patients with inflammatory bowel disease, the gastrointestinal side effects of tirzepatide may be difficult to distinguish from disease flares, and caution is advised, particularly during active disease or if strictures are present.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.