
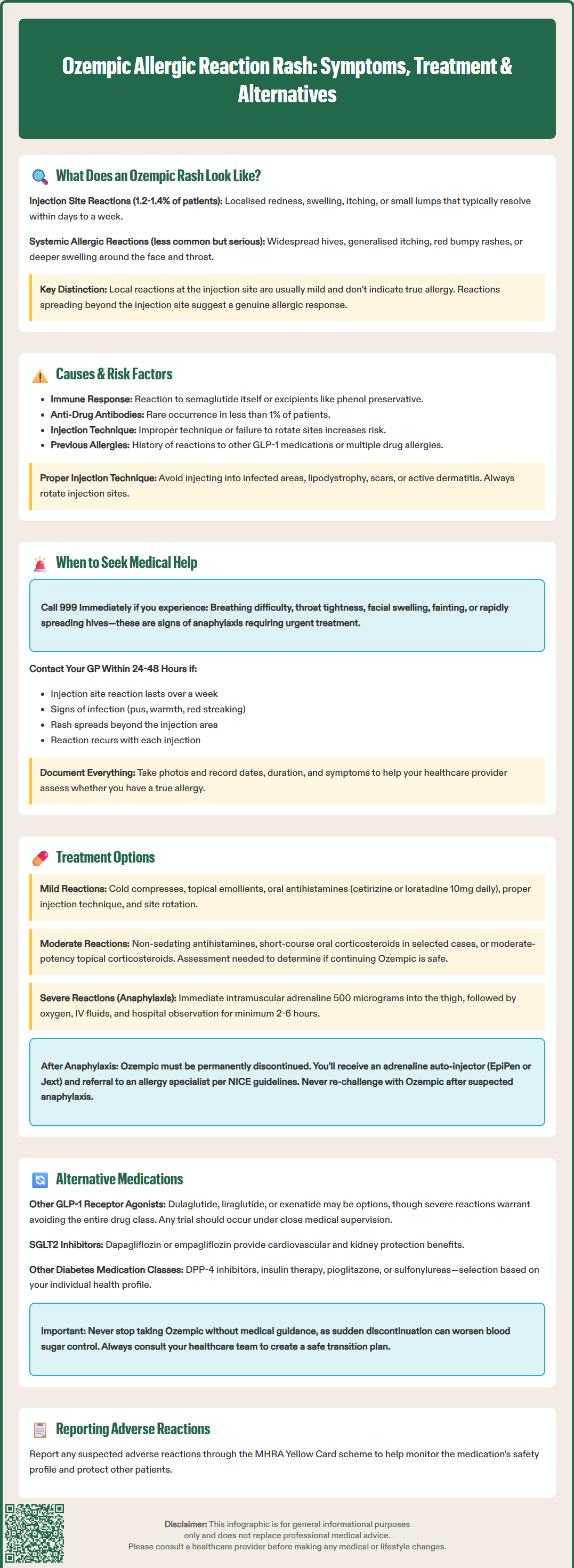
Ozempic allergic reaction rash can range from mild injection site reactions to serious systemic responses requiring urgent medical attention. Ozempic (semaglutide) is a GLP-1 receptor agonist licensed in the UK for type 2 diabetes management. Whilst most patients tolerate the medication well, approximately 1–2% experience skin reactions. These may present as localised redness and swelling at the injection site or, less commonly, as widespread urticaria or angioedema. Understanding the difference between common injection site irritation and genuine allergic reactions is essential for patient safety. This article examines the appearance, causes, and management of Ozempic-related skin reactions, including when to seek medical help and alternative treatment options.
Quick Answer: Ozempic allergic reaction rash typically presents as localised redness, swelling, and itching at the injection site in 1–2% of patients, though rare systemic reactions may cause widespread urticaria or angioedema requiring immediate medical attention.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereOzempic (semaglutide) is a glucagon-like peptide-1 (GLP-1) receptor agonist used for managing type 2 diabetes. In the UK, Ozempic is licensed specifically for type 2 diabetes, while Wegovy (semaglutide 2.4mg) is the licensed formulation for weight management. Whilst most patients tolerate the medication well, skin reactions can occasionally occur at the injection site or more widely across the body.
Injection site reactions are the most commonly reported dermatological adverse effects, occurring in approximately 1.2-1.4% of patients according to the Ozempic SmPC. These typically present as:
Localised redness (erythema) around the injection area
Swelling or raised bumps that may feel warm to touch
Itching or mild discomfort at the site
Small nodules or lumps that can persist for several days
These localised reactions usually appear within hours to days of administration and often resolve spontaneously within a few days to a week. They are generally considered mild and do not necessarily indicate a true allergic response.
Systemic allergic reactions, whilst less common, may manifest as:
Widespread urticaria (hives) – raised, itchy welts that can appear anywhere on the body
Generalised pruritus (itching) without visible rash
Maculopapular rash – flat red areas with small raised bumps
Angioedema – deeper swelling, particularly around the face, lips, or throat
It is important to distinguish between common injection site reactions and genuine allergic responses. True allergic reactions typically involve symptoms beyond the injection site and may include respiratory symptoms, gastrointestinal disturbance, or cardiovascular changes. If you develop a rash accompanied by difficulty breathing, facial swelling, or feeling faint, this constitutes a medical emergency requiring immediate attention.
If a rash spreads beyond the injection site or any systemic symptoms develop, stop further doses and seek clinical advice before administering the next injection.
Understanding the underlying mechanisms behind Ozempic-related skin reactions helps distinguish between different types of responses and their clinical significance.
Pharmacological mechanisms contributing to skin reactions include:
Semaglutide is a synthetic peptide analogue that shares 94% structural homology with native human GLP-1. The medication is formulated with excipients including disodium phosphate dihydrate, propylene glycol, and phenol as a preservative. Skin reactions may result from:
Immune-mediated hypersensitivity to the active semaglutide molecule itself
Reactions to pharmaceutical excipients, particularly phenol or other preservatives
Local tissue irritation from the subcutaneous injection process
Formation of anti-drug antibodies, though this occurs in fewer than 1% of patients according to the EMA European Public Assessment Report (EPAR) for Ozempic
Factors that may potentially increase susceptibility to Ozempic skin reactions include:
Previous allergic reactions to other GLP-1 receptor agonists (such as liraglutide or dulaglutide)
History of atopic conditions – although evidence specifically linking atopy to increased risk of semaglutide reactions is limited
Multiple drug allergies or known hypersensitivity to injectable medications
Improper injection technique, such as injecting too superficially or failing to rotate injection sites
Concurrent skin conditions at the injection site
To minimise injection site reactions, avoid injecting into areas of infection, lipodystrophy, scars or active dermatitis. Proper rotation of injection sites is essential.
It is worth noting that there is no official link established between specific demographic factors (such as age, gender, or ethnicity) and increased risk of allergic reactions to Ozempic. However, patients with a broader history of medication allergies should discuss this with their prescriber before commencing treatment.
Suspected adverse reactions to Ozempic should be reported via the MHRA Yellow Card scheme (yellowcard.mhra.gov.uk or via the Yellow Card app) to help build a comprehensive safety profile.
Determining when a skin reaction requires medical attention is crucial for patient safety. Whilst mild injection site reactions are common and typically self-limiting, certain presentations warrant prompt clinical assessment.
Seek emergency medical attention immediately (call 999 or attend A&E) if you experience:
Sudden breathing difficulty, wheezing, or throat tightness – potential signs of anaphylaxis
Rapid swelling of the face, lips, tongue, or throat (angioedema)
Feeling faint, dizzy, or collapsing – may indicate hypotension
Rapidly progressive widespread urticaria developing after injection
Severe abdominal pain or persistent vomiting
These symptoms may indicate a severe allergic reaction (anaphylaxis), which requires urgent treatment with intramuscular adrenaline and supportive care.
Contact your GP or diabetes specialist nurse within 24–48 hours if:
The injection site reaction persists beyond one week or worsens progressively
You develop signs of infection at the injection site (increasing pain, warmth, pus, or red streaking)
The rash spreads beyond the injection site but without severe symptoms
You experience recurrent reactions with each injection
The reaction significantly impacts your quality of life or ability to continue treatment
If you cannot reach your GP and need urgent advice, contact NHS 111.
Routine monitoring is appropriate for:
Mild redness or swelling at the injection site that resolves within a few days
Slight itching without other symptoms
Small, painless nodules at injection sites that gradually diminish
Keep a record of when reactions occur, their appearance, duration, and any accompanying symptoms. Photographs can be helpful for clinical assessment. Your healthcare provider may need to assess whether the reaction represents a true allergy requiring medication discontinuation or a manageable adverse effect.
Following a suspected allergic reaction to Ozempic, your GP should consider referral to a specialist allergy service in line with NICE Clinical Guideline 183 (Drug allergy: diagnosis and management).
Management of Ozempic-related skin reactions depends on the severity and nature of the presentation. Treatment approaches range from simple supportive measures to medication discontinuation and emergency intervention.
For mild injection site reactions:
Cold compresses applied to the affected area for 10–15 minutes can reduce swelling and discomfort
Topical emollients to soothe irritated skin
Oral antihistamines (such as cetirizine 10mg once daily or loratadine 10mg once daily) may help with itching
Injection technique optimisation – ensure proper rotation of injection sites (abdomen, thigh, or upper arm), inject at room temperature, and use correct subcutaneous technique
Observation – many mild reactions resolve spontaneously without intervention
For moderate allergic reactions:
If you develop urticaria or more widespread rash without severe symptoms, your GP may consider:
Non-sedating antihistamines at standard doses as per BNF guidance
Short course of oral corticosteroids may be prescribed in selected cases after clinical assessment, though not routinely indicated for all skin reactions
Topical corticosteroids (moderate potency) for localised rash
Your healthcare provider will need to assess whether continuing Ozempic is appropriate or whether the medication should be discontinued.
For severe allergic reactions (anaphylaxis):
Emergency treatment includes:
Intramuscular adrenaline (epinephrine) 500 micrograms (0.5ml of 1:1000) into the anterolateral thigh – this is the first-line and most important treatment
High-flow oxygen and airway management
Intravenous fluids for circulatory support
Antihistamines may be given for persistent skin symptoms but are not first-line treatment for anaphylaxis
Hospital observation with duration based on risk assessment (minimum 2-6 hours for uncomplicated cases; longer for severe reactions)
Following a severe allergic reaction, Ozempic must be permanently discontinued, and patients should be provided with an adrenaline auto-injector (such as EpiPen or Jext) if deemed appropriate. In accordance with NICE Clinical Guideline 134, referral to an allergy specialist should be arranged after emergency treatment for suspected anaphylaxis. Do not attempt to re-challenge with Ozempic after a suspected anaphylactic reaction.
If you experience a confirmed allergic reaction to Ozempic, several alternative treatment options are available for managing type 2 diabetes. The choice depends on your individual clinical circumstances, treatment goals, and previous medication history, in line with NICE guideline NG28 (Type 2 diabetes in adults: management).
Alternative GLP-1 receptor agonists:
Whilst cross-reactivity between different GLP-1 receptor agonists is possible, some patients who react to one agent may tolerate another. Options include:
Dulaglutide (Trulicity) – once-weekly injection with a different molecular structure
Liraglutide (Victoza) – once-daily injection
Exenatide (Byetta, Bydureon) – twice-daily or once-weekly formulations
However, if the allergic reaction was severe, many clinicians would advise avoiding the entire drug class. Any trial of an alternative GLP-1 agonist should occur under close medical supervision, ideally in a setting where emergency treatment is readily available.
Oral GLP-1 receptor agonist:
Alternative medication classes for type 2 diabetes:
NICE guideline NG28 recommends individualised treatment approaches. Alternatives include:
SGLT2 inhibitors (such as dapagliflozin, empagliflozin, or canagliflozin) – offer cardiovascular and renal benefits; selection should consider presence of cardiovascular disease, heart failure, or chronic kidney disease
DPP-4 inhibitors (such as sitagliptin or linagliptin) – oral agents with a different mechanism
Insulin therapy – particularly if glycaemic control is inadequate with oral agents
Pioglitazone – may be appropriate in selected patients without heart failure
Sulfonylureas (such as gliclazide) – though associated with hypoglycaemia and weight gain
Your diabetes specialist or GP will conduct a comprehensive review of your treatment plan, considering factors such as HbA1c targets, cardiovascular risk profile, renal function, and personal preferences. It is essential not to discontinue Ozempic without medical guidance, as abrupt cessation may lead to deterioration in glycaemic control. Always discuss alternative options with your healthcare team to ensure safe and effective diabetes management tailored to your individual needs.
Mild injection site redness and swelling that resolves within days is common and usually not serious. Seek emergency help immediately if you develop facial swelling, breathing difficulty, widespread hives, or feel faint, as these indicate a severe allergic reaction requiring urgent treatment.
Mild, localised injection site reactions that resolve within a week often do not require stopping treatment. However, if the rash spreads beyond the injection site, persists beyond one week, or you develop systemic symptoms, stop further doses and contact your GP or diabetes specialist before the next injection.
Alternatives include other GLP-1 receptor agonists (such as dulaglutide or liraglutide), SGLT2 inhibitors (such as dapagliflozin or empagliflozin), DPP-4 inhibitors, or insulin therapy. Your diabetes specialist will recommend the most appropriate option based on your individual clinical circumstances and treatment goals in line with NICE guideline NG28.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.