
'Ozempic feet' is an informal term used in patient communities to describe foot-related symptoms—such as pain, swelling, or tingling—reported by some individuals taking Ozempic (semaglutide), a GLP-1 receptor agonist licensed in the UK for type 2 diabetes. Whilst not an officially recognised adverse effect in regulatory documentation from the MHRA or EMA, anecdotal reports have prompted questions about potential links. Many patients taking Ozempic have type 2 diabetes, a condition independently associated with peripheral neuropathy and foot complications. This article examines the evidence, symptoms, and appropriate management strategies for foot health during semaglutide therapy.
Quick Answer: 'Ozempic feet' is an informal, non-medical term describing foot symptoms such as pain, swelling, or tingling reported by some patients taking semaglutide, though no causal link is established in clinical data.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start Here'Ozempic feet' is an informal term that has emerged in patient communities and social media to describe various foot-related symptoms some individuals report whilst taking Ozempic (semaglutide). Ozempic is a glucagon-like peptide-1 (GLP-1) receptor agonist licensed in the UK for the treatment of type 2 diabetes mellitus, while a higher-dose formulation (Wegovy) is licensed for weight management. Ozempic works by mimicking the action of the naturally occurring hormone GLP-1, which stimulates glucose-dependent insulin secretion, suppresses glucagon release, and slows gastric emptying.
Whilst 'Ozempic feet' is not an officially recognised medical diagnosis or listed adverse effect in the Summary of Product Characteristics (SmPC), anecdotal reports have described symptoms such as foot pain, swelling, tingling, or changes in foot appearance during treatment. It is important to note that there is no established causal link between semaglutide and specific foot pathology in clinical trial data or regulatory documentation from the MHRA or EMA.
Many patients taking Ozempic have type 2 diabetes, a condition independently associated with peripheral neuropathy, peripheral arterial disease, and increased risk of foot complications. Some have hypothesised that rapid weight loss—a common effect of semaglutide therapy—might alter foot biomechanics or fat pad distribution, potentially contributing to discomfort, though this remains speculative without specific evidence. Similarly, while dehydration from gastrointestinal side effects (nausea, vomiting, diarrhoea) could theoretically affect fluid balance, direct effects on peripheral circulation or nerve function are not established in the literature.
Patients experiencing new or worsening foot symptoms whilst on Ozempic should not assume these are directly caused by the medication. A thorough clinical assessment is essential to identify underlying causes, which may include pre-existing diabetic complications, musculoskeletal issues, or unrelated conditions requiring specific management.
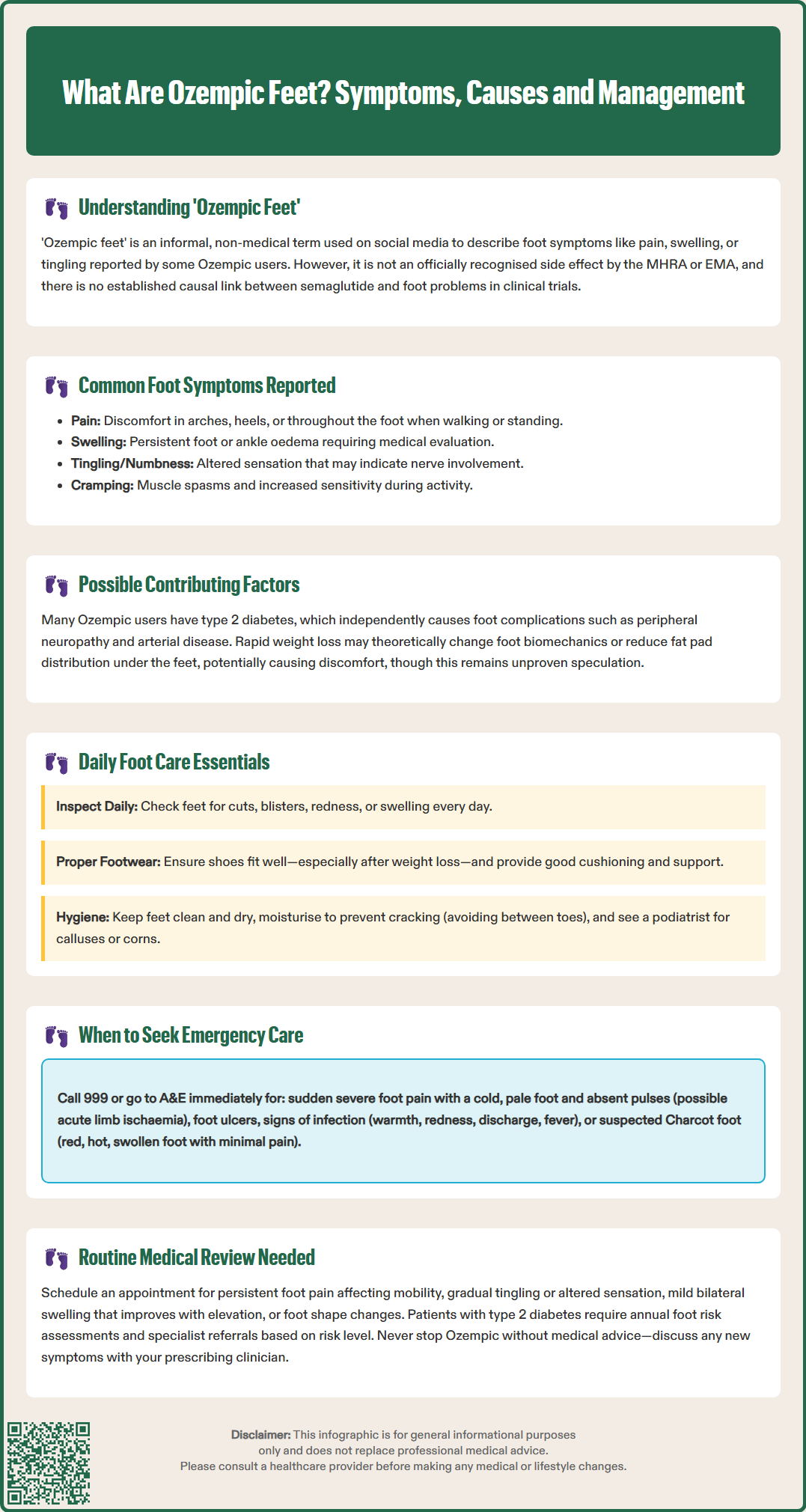
Individuals reporting foot symptoms whilst taking Ozempic describe a range of presentations, though these are not unique to semaglutide therapy. Common complaints include:
Pain or aching in the feet, particularly in the arches, heels, or balls of the feet
Swelling (oedema) of the feet or ankles, which may worsen throughout the day
Tingling, numbness, or 'pins and needles' sensations, suggestive of peripheral neuropathy
Changes in foot shape or appearance, potentially related to weight loss
Increased sensitivity or discomfort when walking or standing
Cramping in the feet or lower legs, particularly at night
It is crucial to distinguish between symptoms that may be coincidental and those requiring urgent attention. Diabetic foot complications—including neuropathy, peripheral arterial disease, and ulceration—remain significant concerns for patients with type 2 diabetes, regardless of their medication regimen. The presence of reduced sensation, absent pulses, skin colour changes (pallor, cyanosis, or rubor), non-healing wounds, or signs of infection warrants immediate medical evaluation.
Rapid weight loss associated with GLP-1 receptor agonist therapy may hypothetically alter foot biomechanics. Some clinicians have suggested that loss of plantar fat padding could increase pressure on bony prominences, potentially leading to discomfort or callus formation, though this mechanism requires further study.
While some patients report fluid retention in the lower extremities, it should be noted that peripheral oedema is not specifically listed as an adverse effect in the current UK Ozempic SmPC. Patients with swelling should be assessed for cardiac, renal, or hepatic causes of oedema, particularly if swelling is persistent, unilateral, or accompanied by breathlessness or significant weight gain.
Proactive foot care is essential for all patients with type 2 diabetes, and this remains paramount for those taking Ozempic. NICE guidance (NG19) on diabetic foot problems emphasises the importance of annual foot risk assessment and patient education regarding foot care. This includes risk stratification (low, moderate, high, or active foot problem) with appropriate referral to foot protection services or multidisciplinary foot care services based on risk level.
Key self-care measures include:
Daily foot inspection: Examine feet for cuts, blisters, redness, swelling, or changes in skin integrity. Use a mirror or ask a family member to check areas that are difficult to see.
Appropriate footwear: Ensure shoes fit properly, particularly if weight loss has occurred. Avoid tight, restrictive footwear and opt for supportive shoes with adequate cushioning. Consider professional fitting or orthotics if foot pain develops.
Maintain skin integrity: Keep feet clean and dry, moisturise to prevent cracking (avoiding between the toes), and address calluses or corns with professional podiatry input rather than self-treatment.
Gradual increase in activity: If increasing physical activity as part of diabetes management, do so progressively to allow feet to adapt and reduce injury risk.
Addressing medication-related factors:
If peripheral oedema develops, elevating the legs when resting and ensuring adequate hydration may provide symptomatic relief. However, persistent, worsening, or unilateral swelling requires prompt medical review to exclude serious causes such as deep vein thrombosis or infection.
Patients experiencing gastrointestinal side effects should maintain adequate fluid intake, as dehydration may exacerbate general discomfort. If nausea or vomiting is severe, dose adjustment or antiemetic therapy may be appropriate.
Regular monitoring of glycaemic control, blood pressure, and lipid profiles—as per standard diabetes care pathways—helps reduce long-term vascular and neuropathic complications. Optimising overall diabetes management remains the most effective strategy for preventing diabetic foot disease.
Patients who believe they have experienced side effects from Ozempic should report these through the MHRA Yellow Card Scheme (yellowcard.mhra.gov.uk).
Patients should be advised to contact their GP or diabetes care team promptly if they experience any of the following:
Emergency (999/A&E attendance):
Urgent referral indicators (same-day assessment to multidisciplinary foot care service):
Ulceration or breaks in skin integrity (any suspected diabetic foot ulcer)
Signs of infection: warmth, redness, swelling, discharge, or systemic symptoms (fever, malaise)
Severe pain, particularly if sudden in onset or associated with colour changes
Suspected acute Charcot foot: red, hot, swollen foot, often with minimal pain in the presence of neuropathy
Ischaemic signs: cold feet, absent pulses, pallor, or pain at rest
Unilateral, painful swelling (possible deep vein thrombosis)
Routine review appropriate for:
Persistent foot pain or discomfort affecting mobility or quality of life
Gradual onset of tingling or altered sensation
Bilateral, mild swelling that resolves with elevation
Concerns about foot shape changes or difficulty finding appropriate footwear
Development of calluses, corns, or areas of pressure
Patients with established diabetic neuropathy or peripheral arterial disease should have enhanced surveillance and may require referral to specialist podiatry or multidisciplinary diabetic foot services, in line with NICE NG19 recommendations. Those assessed as having moderate or high risk of foot problems should be under regular review by the foot protection service.
If foot symptoms develop shortly after starting or increasing the dose of Ozempic, this should be discussed with the prescribing clinician. Whilst a direct causal relationship is not established, a comprehensive assessment can identify contributing factors and guide appropriate management. In some cases, dose adjustment, additional investigations (such as nerve conduction studies or vascular assessment), or alternative therapeutic strategies may be considered.
Patients should never discontinue Ozempic without medical advice, as abrupt cessation may affect glycaemic control. Any concerns about medication tolerability should be addressed through shared decision-making with healthcare professionals.
No, 'Ozempic feet' is not listed as an adverse effect in the UK Summary of Product Characteristics or regulatory documentation from the MHRA or EMA. It is an informal term used in patient communities to describe various foot symptoms.
Seek urgent assessment for ulceration, signs of infection (warmth, redness, discharge), severe pain, cold or pale feet with absent pulses, or unilateral painful swelling. These may indicate serious diabetic foot complications requiring immediate multidisciplinary care.
Inspect feet daily for cuts or changes, wear properly fitted supportive footwear, maintain skin integrity with moisturisation, and attend annual foot risk assessments as recommended by NICE guidance (NG19). Report any new symptoms to your diabetes care team promptly.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.