
Using expired Mounjaro (tirzepatide) is not recommended, as the medication may have lost potency or compromised sterility beyond its expiry date. Mounjaro is a dual GIP and GLP-1 receptor agonist prescribed for type 2 diabetes management in the UK. Whilst expired Mounjaro is unlikely to cause immediate harm, reduced effectiveness can lead to inadequate blood glucose control, undermining your diabetes treatment. This article explains what happens if you use expired Mounjaro, how to check expiry dates, proper storage guidelines, and safe disposal procedures to ensure optimal medication safety and therapeutic outcomes.
Quick Answer: Using expired Mounjaro primarily reduces its effectiveness rather than causing immediate harm, potentially leading to inadequate blood glucose control.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereMounjaro (tirzepatide) is a prescription medicine used to improve blood sugar control in adults with type 2 diabetes mellitus. As a glucagon-like peptide-1 (GLP-1) receptor agonist and glucose-dependent insulinotropic polypeptide (GIP) receptor agonist, it works by stimulating insulin secretion, suppressing glucagon release, and slowing gastric emptying. Like all medicines, Mounjaro has an expiry date printed on its packaging, which indicates the last date the manufacturer guarantees full potency and safety when stored correctly.
Expiry dates are determined through rigorous stability testing conducted by pharmaceutical manufacturers and reviewed as part of the marketing authorisation approved by the Medicines and Healthcare products Regulatory Agency (MHRA). These dates ensure that the active ingredient—tirzepatide—remains at its specified concentration and that the formulation maintains its sterility and chemical integrity. Using medication beyond its expiry date is not recommended, as the medicine may have degraded, potentially reducing its effectiveness or altering its safety profile.
For injectable medicines like Mounjaro, which come in pre-filled pens, maintaining product integrity is particularly important. It's essential to understand that Mounjaro pens are single-dose devices that must be discarded immediately after one injection. The expiry date applies to unopened pens stored under recommended conditions.
Key considerations include:
The expiry date refers to the end of the stated month
Proper refrigeration is essential for unopened pens
Unopened pens may be kept at room temperature (≤30°C) for up to 21 days
Never use Mounjaro if it appears cloudy, discoloured, or contains particles
Patients should always check expiry dates before administration and consult their GP or pharmacist if uncertain about their medication's status, as outlined in the Mounjaro Patient Information Leaflet.
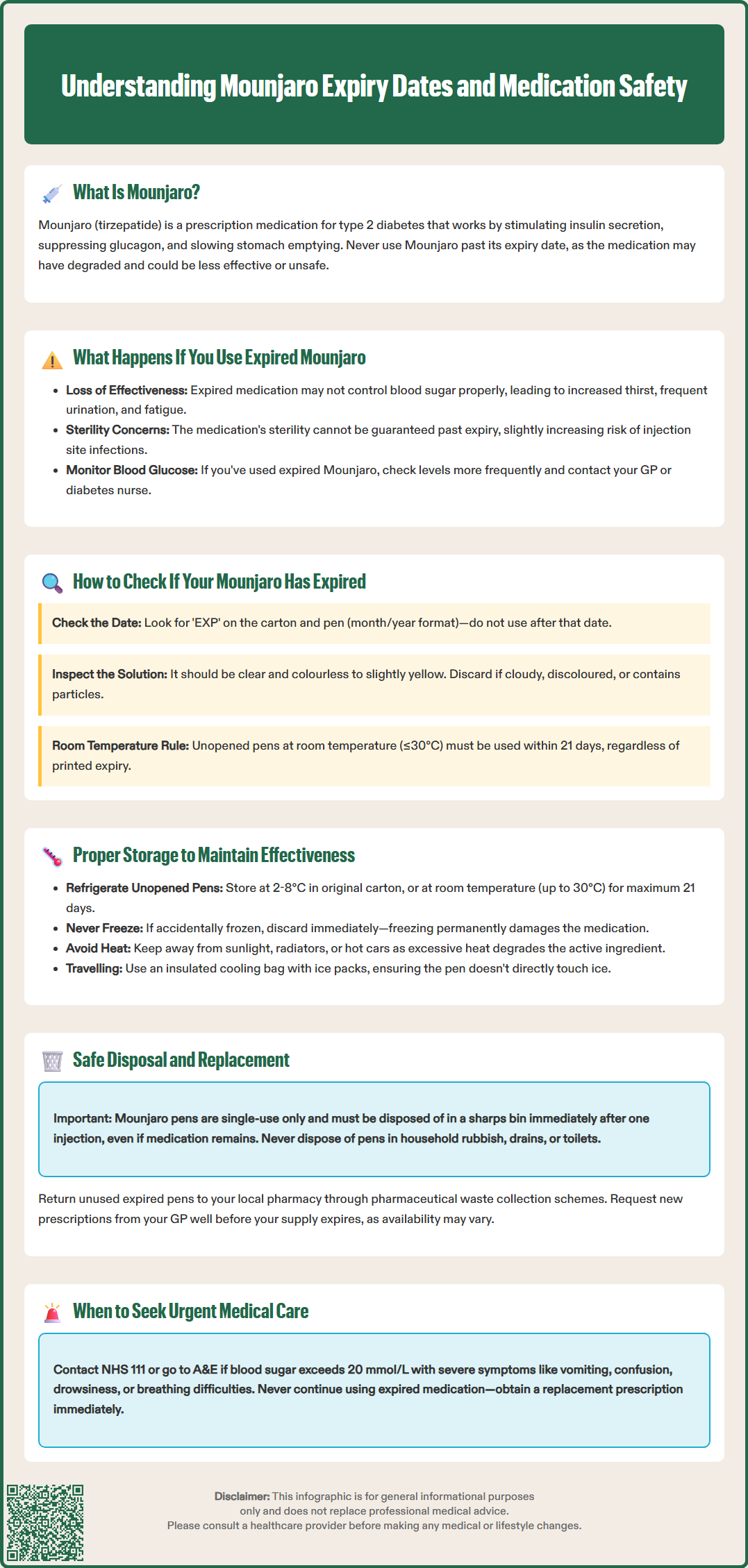
Using expired Mounjaro may result in reduced therapeutic effectiveness rather than immediate harm, though the exact consequences depend on how long past expiry the medication is and how it has been stored. The primary concern is that tirzepatide, the active pharmaceutical ingredient, may have degraded over time, meaning the dose you administer may contain less active medicine than intended. This could lead to inadequate blood glucose control, potentially causing hyperglycaemia (elevated blood sugar levels) and undermining your diabetes management.
Whilst there is no official evidence suggesting that expired Mounjaro becomes toxic or causes serious adverse reactions, the sterility of the solution cannot be guaranteed beyond the expiry date. Injectable medicines must remain sterile to prevent infection at the injection site. Degradation of preservatives or changes in the formulation's pH could theoretically increase the risk of local reactions, though such occurrences are not well-documented in clinical literature.
Potential consequences of using expired Mounjaro include:
Reduced efficacy: Inadequate blood glucose control due to decreased potency
Unpredictable dosing: Uncertainty about the actual amount of active ingredient delivered
Possible injection site reactions: Though rare, compromised formulation integrity may increase local irritation
Loss of confidence in treatment: Inconsistent results may affect adherence to therapy
If you have inadvertently used expired Mounjaro, monitor your blood glucose levels more frequently and watch for signs of poor diabetes control, such as increased thirst, frequent urination, fatigue, or blurred vision. Contact your GP or diabetes specialist nurse promptly to discuss the situation. They may recommend additional blood glucose monitoring or adjustments to your treatment plan.
Important: If your blood glucose remains very high (e.g., above 20 mmol/L) with symptoms such as dehydration, vomiting, drowsiness, confusion, or breathing difficulties, seek urgent medical advice via NHS 111 or attend A&E. Do not simply continue using expired medication—obtain a replacement prescription as soon as possible to ensure optimal diabetes management and patient safety.
Checking whether your Mounjaro has expired is straightforward and should become part of your routine before each injection. The expiry date is clearly printed on both the outer carton and on each individual pen. It typically appears as 'EXP' followed by a month and year (e.g., EXP 08/2025), indicating that the medicine should not be used after the last day of that month. Always examine the packaging carefully in good lighting to avoid misreading the date.
Beyond checking the printed expiry date, you should also perform a visual inspection of the Mounjaro solution before each use. According to the Mounjaro Patient Information Leaflet, the medicine should be clear and colourless to slightly yellow. If the solution appears cloudy, discoloured, or contains visible particles or crystals, do not use it, regardless of the expiry date. These changes indicate that the formulation has degraded or been compromised, potentially due to improper storage or temperature exposure.
Steps to verify your Mounjaro is safe to use:
Check the outer carton: Locate the expiry date before removing the pen
Inspect the pen label: Confirm the date matches what's on the carton
Examine the solution: Hold the pen up to light and check for clarity
Consider storage history: Assess whether the pen has been exposed to heat or freezing
Remember: Unopened pens kept at room temperature (≤30°C) should be used within 21 days
If you discover that your Mounjaro has expired or you're uncertain about its status, do not use it. Contact your pharmacy to obtain a replacement. Many pharmacies maintain records of your prescriptions and can help arrange a new supply. If you're running low on in-date medication, speak with your GP surgery promptly to arrange a new prescription, ensuring continuity of your diabetes treatment without resorting to expired medicines.
Remember that Mounjaro pens are single-dose devices and must be disposed of in a sharps bin immediately after one injection.
Correct storage of Mounjaro is essential to maintain its potency and safety throughout its shelf life. According to the MHRA-approved Summary of Product Characteristics (SmPC), unopened Mounjaro pens must be stored in a refrigerator at temperatures between 2°C and 8°C, protected from light. Store the pens in their original carton to shield them from light exposure, which can degrade the active ingredient. Never store Mounjaro in the freezer, and if a pen has been accidentally frozen, it must be discarded, as freezing irreversibly damages the formulation.
The SmPC also states that an unopened pen may be kept at room temperature (not exceeding 30°C) for up to 21 days. Many patients find room temperature storage more comfortable, as injecting cold medication can be uncomfortable. However, the pen must still be protected from direct heat and light. Never leave Mounjaro in direct sunlight, near radiators, or in hot environments such as cars, as excessive heat accelerates degradation.
Best practices for Mounjaro storage:
Unopened pens: Refrigerate at 2–8°C in the original carton
Alternative for unopened pens: May be kept at room temperature (≤30°C) for up to 21 days
Never freeze: Discard if accidentally frozen
Protect from light: Keep in carton when not in use
Single-use only: Mounjaro pens are for one injection only and must be disposed of immediately after use in a sharps bin
Keep away from children: Store securely in a safe location
When travelling, use an insulated cooling bag with ice packs (ensuring the pen doesn't touch ice directly) to maintain appropriate temperatures. If you're unsure whether your Mounjaro has been exposed to inappropriate temperatures, consult your pharmacist. Proper storage not only prevents premature expiry but ensures each dose delivers the intended therapeutic effect, supporting optimal diabetes control and reducing the risk of complications. Following the storage guidelines in the Mounjaro Patient Information Leaflet protects both the medicine's integrity and your health.
Disposing of expired or unused Mounjaro correctly is crucial for environmental protection and public safety. Never throw Mounjaro pens in household rubbish or pour the contents down drains or toilets, as this can contaminate water supplies and harm wildlife. Injectable medicines require special disposal procedures to prevent accidental needle-stick injuries and ensure pharmaceutical waste doesn't enter the environment.
There are different disposal methods depending on whether the pen has been used:
For unused expired Mounjaro pens:
Return to your local pharmacy, which can accept unused medicines as part of pharmaceutical waste collection schemes
Keep in the original carton if possible to help pharmacy staff identify the medicine
For used Mounjaro pens:
Dispose of immediately after a single use in a proper sharps bin (never recap the needle)
Obtain a sharps bin from your GP, diabetes clinic, or pharmacy on prescription
Follow local council arrangements for sharps bin collection or return to designated NHS facilities
Never place used pens in regular household waste or recycling
It's important to note that not all pharmacies accept used sharps containers—check your local council website or NHS services for specific sharps disposal arrangements in your area.
To obtain replacement Mounjaro, contact your GP surgery to request a new prescription well before your current supply expires. Most surgeries allow prescription requests online, by phone, or in person. Your GP may require a review appointment, particularly if you're approaching the end of a prescription cycle or if your diabetes control needs assessment. Once issued, take your prescription to your nominated pharmacy. Availability of Mounjaro may vary, and there could be supply constraints, so allow sufficient time for your prescription to be processed.
If you've run out of Mounjaro unexpectedly, contact your GP surgery immediately, explaining the situation. They may issue an emergency prescription or provide interim advice. Never ration doses or skip injections without medical guidance, as this can compromise your diabetes control. Maintaining a regular supply through timely prescription requests ensures continuous, effective treatment whilst avoiding the temptation to use expired medication.
Expired Mounjaro is unlikely to cause immediate harm, but its reduced potency may lead to inadequate blood glucose control. Sterility cannot be guaranteed beyond the expiry date, though serious adverse reactions are not well-documented.
You should not use Mounjaro after the expiry date, as the manufacturer cannot guarantee full potency or safety beyond that point. Contact your GP or pharmacy immediately for a replacement prescription.
Return unused expired Mounjaro pens to your local pharmacy as part of pharmaceutical waste schemes. Used pens must be disposed of in sharps bins, which can be obtained from your GP or pharmacy and returned according to local NHS arrangements.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.