
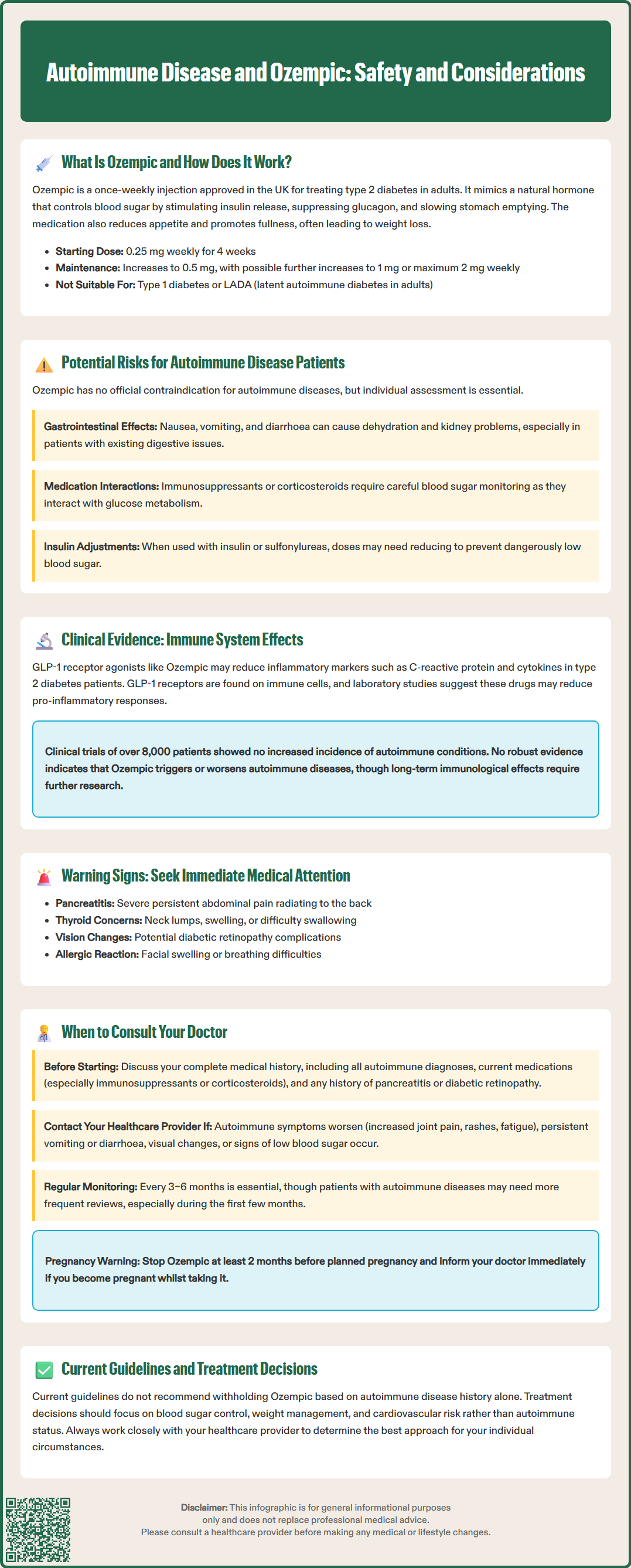
Ozempic (semaglutide) is a glucagon-like peptide-1 (GLP-1) receptor agonist licensed in the UK for treating type 2 diabetes mellitus in adults. For patients with autoimmune diseases—conditions where the immune system attacks the body's own tissues—questions often arise about whether Ozempic is safe and appropriate. Whilst there is no official contraindication to using Ozempic in autoimmune disease patients, careful consideration of individual circumstances, potential interactions with immunosuppressive therapies, and monitoring for side effects remain essential. This article explores the relationship between autoimmune disease and Ozempic, examining clinical evidence, safety considerations, and when to seek medical advice.
Quick Answer: Ozempic is not contraindicated in autoimmune disease patients, but individualised assessment and monitoring are essential before and during treatment.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereOzempic (semaglutide) is a prescription medicine licensed in the UK for the treatment of type 2 diabetes mellitus in adults. It belongs to a class of medications called glucagon-like peptide-1 (GLP-1) receptor agonists, which work by mimicking the action of a naturally occurring hormone in the body.
The mechanism of action involves several complementary pathways. Semaglutide binds to GLP-1 receptors on pancreatic beta cells, stimulating insulin secretion in a glucose-dependent manner—meaning it only triggers insulin release when blood glucose levels are elevated. Simultaneously, it suppresses the release of glucagon, a hormone that raises blood sugar. This dual action helps maintain more stable glycaemic control throughout the day.
Beyond its effects on pancreatic hormones, Ozempic also slows gastric emptying, which reduces the rate at which food enters the small intestine. This contributes to improved post-meal glucose levels and promotes a feeling of fullness. Additionally, semaglutide acts on appetite centres in the brain, reducing hunger and food intake, which often leads to weight loss—a beneficial effect for many people with type 2 diabetes.
Ozempic is administered as a once-weekly subcutaneous injection using a pre-filled pen device. Treatment starts with 0.25 mg once weekly for 4 weeks (for dose initiation only, not for glycaemic control), then increases to 0.5 mg once weekly. After at least 4 weeks, the dose can be increased to 1 mg weekly and, if necessary, to a maximum of 2 mg weekly for additional glycaemic control.
Importantly, Ozempic is not indicated for type 1 diabetes or diabetic ketoacidosis and is not a substitute for insulin. When used with insulin or sulfonylureas (e.g., gliclazide), dose reductions of these medications may be required to reduce the risk of hypoglycaemia.
The Medicines and Healthcare products Regulatory Agency (MHRA) has approved Ozempic specifically for glycaemic control in type 2 diabetes, either as monotherapy when metformin is inappropriate or in combination with other glucose-lowering medications. Whilst weight loss is a common secondary benefit, a separate formulation (Wegovy, also semaglutide) is licensed specifically for weight management in the UK.
Patients with autoimmune diseases require careful consideration before starting any new medication, including Ozempic. Autoimmune conditions occur when the immune system mistakenly attacks the body's own tissues, and individuals with one autoimmune disease are at increased risk of developing others—a phenomenon known as polyautoimmunity.
Currently, there is no official contraindication to using Ozempic in patients with autoimmune diseases. The UK Summary of Product Characteristics (SmPC) lists only hypersensitivity to semaglutide or any excipients as a contraindication. However, certain considerations warrant discussion with your healthcare provider:
Type 1 diabetes and latent autoimmune diabetes in adults (LADA): Ozempic is not licensed for type 1 diabetes. Patients with LADA usually require insulin therapy, often early in their disease course, though timing varies between individuals.
Thyroid considerations: The prescribing information includes a precautionary warning about thyroid C-cell tumours observed in rodent studies, though the relevance to humans remains uncertain. Patients should report any symptoms such as a neck mass, difficulty swallowing, or persistent hoarseness to their healthcare provider.
Gastrointestinal disease: The UK SmPC notes limited experience in patients with severe gastrointestinal disease, including gastroparesis. Ozempic commonly causes nausea, vomiting, and diarrhoea, particularly during dose escalation, which may lead to dehydration and acute kidney injury in susceptible individuals.
Other important safety considerations: Patients should be aware of the risk of acute pancreatitis (report severe, persistent abdominal pain), diabetic retinopathy complications (particularly in those with pre-existing retinopathy on insulin), and gallbladder disease. Dehydration from gastrointestinal side effects may affect renal function, so adequate fluid intake is important.
Immunosuppressive therapy: Many autoimmune disease patients take immunosuppressants or corticosteroids, which can affect glucose metabolism. Careful monitoring of blood glucose is essential when combining these treatments with Ozempic.
The relationship between GLP-1 receptor agonists like Ozempic and the immune system is an area of ongoing research, with emerging evidence suggesting potential immunomodulatory effects beyond glucose control.
Several studies have investigated whether semaglutide influences inflammatory markers. Research published in diabetes and cardiovascular journals has demonstrated that GLP-1 receptor agonists may reduce levels of C-reactive protein (CRP) and other inflammatory cytokines in patients with type 2 diabetes. Some studies suggest these anti-inflammatory properties may be partially independent of weight loss, though findings are mixed and the clinical significance remains unclear.
Preclinical and laboratory studies have shown that GLP-1 receptors are expressed not only in pancreatic tissue but also on various immune cells, including macrophages, lymphocytes, and dendritic cells. These experimental data suggest that GLP-1 receptor activation may modulate immune cell function, potentially reducing pro-inflammatory responses. However, it's important to note that evidence in autoimmune disease populations specifically remains limited, with no randomised controlled trials targeting autoimmune disease outcomes with semaglutide.
Importantly, there is no robust evidence that Ozempic triggers or worsens autoimmune diseases. Post-marketing surveillance data and clinical trials have not identified an increased incidence of new-onset autoimmune conditions in patients treated with semaglutide. The SUSTAIN clinical trial programme, which evaluated Ozempic in over 8,000 patients with type 2 diabetes, did not report autoimmune disease development as a safety concern.
Nevertheless, the long-term immunological effects of GLP-1 receptor agonists require further investigation. Current evidence does not support withholding Ozempic solely based on autoimmune disease history, but individualised risk-benefit assessment remains essential. The National Institute for Health and Care Excellence (NICE) guideline NG28 recommends GLP-1 receptor agonists as part of type 2 diabetes management pathways, with treatment decisions based on glycaemic control, weight, and cardiovascular risk factors rather than autoimmune status.
If you have an autoimmune disease and are considering Ozempic, or if you develop concerning symptoms whilst taking it, prompt medical consultation is essential. Open communication with your healthcare team ensures safe, personalised treatment.
Before starting Ozempic, discuss your complete medical history with your GP or diabetes specialist, including:
All diagnosed autoimmune conditions and their current activity status
Medications you take for autoimmune disease management, particularly immunosuppressants or corticosteroids
Any history of pancreatitis, severe gastrointestinal disease, or diabetic retinopathy
Previous adverse reactions to diabetes medications
Your doctor will assess whether Ozempic is appropriate and may recommend baseline investigations, such as renal function and HbA1c measurement. If you have pre-existing diabetic retinopathy, an eye assessment may be advised.
During treatment, contact your healthcare provider if you experience:
Severe or persistent abdominal pain, particularly if radiating to the back, which could indicate pancreatitis
Neck swelling or lump, difficulty swallowing, or persistent hoarseness
Worsening symptoms of your autoimmune condition, such as increased joint pain, rashes, or fatigue
Severe gastrointestinal symptoms including persistent vomiting or diarrhoea, which may lead to dehydration and affect kidney function
Signs of hypoglycaemia (low blood sugar), especially if taking Ozempic with insulin or sulfonylureas
Visual changes or symptoms suggesting diabetic retinopathy progression
Signs of severe allergic reaction such as swelling of the face, lips, tongue, or throat, breathing difficulties, or severe rash (seek urgent medical attention)
Regular monitoring is crucial. NICE guidance recommends reviewing diabetes treatment every 3–6 months, assessing glycaemic control, weight, tolerability, and any complications. Patients with autoimmune diseases may benefit from more frequent reviews, particularly during the initial months of treatment.
If you're planning pregnancy, Ozempic should be discontinued at least 2 months before a planned conception. If you become pregnant whilst taking Ozempic, contact your healthcare team immediately, as semaglutide is not recommended during pregnancy. Similarly, inform your doctor before any planned surgery, as temporary discontinuation may be recommended following UK perioperative guidance due to Ozempic's effects on gastric emptying.
Your multidisciplinary team—including your GP, diabetes specialist, and rheumatologist or other relevant specialists—should coordinate your care to optimise both diabetes management and autoimmune disease control.
If you experience any suspected side effects from Ozempic, report them to the MHRA Yellow Card Scheme, which helps monitor the safety of medicines in the UK.
There is no official contraindication to using Ozempic in patients with autoimmune diseases. However, individualised assessment by your healthcare provider is essential, particularly if you take immunosuppressants or corticosteroids, as these may affect blood glucose control.
Current evidence does not suggest that Ozempic triggers or worsens autoimmune diseases. Clinical trials involving over 8,000 patients did not identify increased incidence of new-onset autoimmune conditions, though long-term immunological effects require further research.
Monitor for severe abdominal pain, neck swelling, worsening autoimmune symptoms, persistent gastrointestinal side effects, and signs of hypoglycaemia. Regular reviews every 3–6 months assessing glycaemic control, weight, and tolerability are recommended, with more frequent monitoring for autoimmune disease patients.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.