
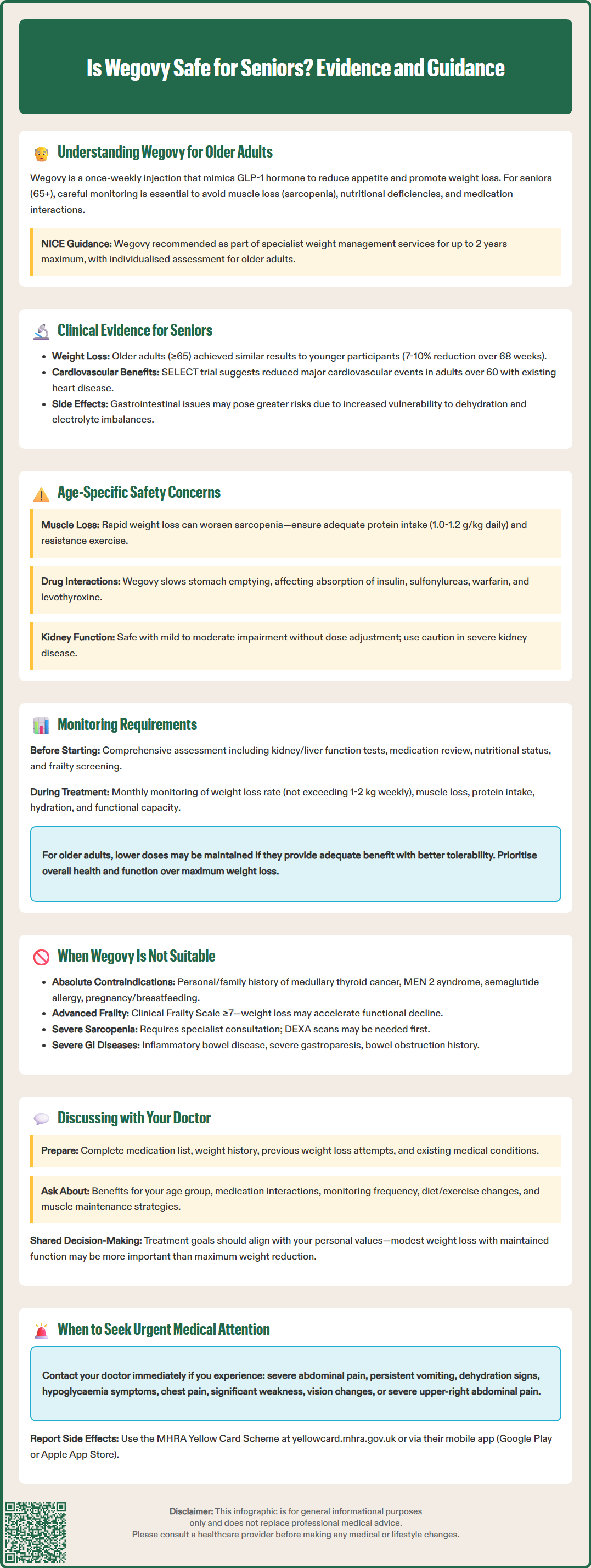
Wegovy (semaglutide 2.4 mg) is increasingly prescribed for weight management in the UK, but is Wegovy safe for seniors? As a GLP-1 receptor agonist licensed by the MHRA, Wegovy offers effective weight loss for adults with obesity or overweight with comorbidities. However, older adults—typically those aged 65 and above—present unique considerations including multiple health conditions, polypharmacy, and age-related changes affecting drug metabolism. Whilst clinical evidence suggests comparable efficacy in seniors, careful assessment of frailty, sarcopenia risk, and individual health goals is essential before initiating treatment in this population.
Quick Answer: Wegovy can be safe for seniors when carefully selected and monitored, though older adults require individualised assessment for frailty, sarcopenia risk, and medication interactions.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereWegovy (semaglutide 2.4 mg) is a glucagon-like peptide-1 (GLP-1) receptor agonist licensed by the MHRA for weight management in adults with obesity (BMI ≥30 kg/m²) or overweight (BMI ≥27 kg/m²) with weight-related comorbidities. As the UK population ages, an increasing number of older adults are seeking effective weight management solutions, raising important questions about the safety and appropriateness of Wegovy for seniors.
Mechanism of action: Wegovy works by mimicking the naturally occurring hormone GLP-1, which regulates appetite and food intake. It slows gastric emptying, enhances satiety signals to the brain, and reduces hunger. This leads to decreased caloric intake and subsequent weight loss. The medication is administered once weekly via subcutaneous injection, offering convenience for patients who may struggle with daily medication regimens.
Older adults—typically defined as those aged 65 years and above—often present with multiple comorbidities, polypharmacy, and age-related physiological changes that can influence drug metabolism and tolerability. Whilst obesity in later life is associated with increased cardiovascular risk, type 2 diabetes, and reduced mobility, weight loss interventions must be carefully balanced against potential risks such as sarcopenia (muscle loss), nutritional deficiencies, and medication interactions.
Current guidance: NICE Technology Appraisal 875 recommends Wegovy as part of a specialist weight management service for appropriate patients meeting specific criteria. Treatment is limited to a maximum of 2 years. However, specific guidance for older adults requires individualised assessment, taking into account frailty status, functional capacity, and overall health goals. The decision to prescribe Wegovy to seniors should involve shared decision-making between patient and clinician.
Clinical trial data for Wegovy includes participants across various age groups, though older adults have been underrepresented in pivotal studies. The STEP (Semaglutide Treatment Effect in People with obesity) clinical trial programme included participants up to 75 years of age, providing some evidence for safety and efficacy in older populations, though the majority of participants were younger than 65.
Key safety findings: In subgroup analyses of STEP trials, older adults (≥65 years) experienced similar weight loss benefits to younger participants, with placebo-adjusted weight reductions of around 7-10% of baseline body weight over 68 weeks. However, the incidence of gastrointestinal adverse effects—including nausea, vomiting, diarrhoea, and constipation—was comparable across age groups. These side effects, whilst generally mild to moderate and transient, may be more problematic for older adults who are at higher risk of dehydration and electrolyte imbalances.
Preliminary cardiovascular safety data from the SELECT trial (Semaglutide Effects on Heart Disease and Stroke in Patients with Overweight or Obesity) suggest that semaglutide may reduce major adverse cardiovascular events in adults with established cardiovascular disease. This trial included a substantial proportion of participants over 60 years, potentially indicating cardiovascular benefits for older adults with obesity and heart disease, though these findings are not yet reflected in the product licence.
Adverse effects of particular concern in seniors include:
Gastrointestinal disturbances leading to reduced nutritional intake
Potential exacerbation of gastroparesis in those with diabetic complications
Rare cases of acute pancreatitis
Possible increased risk of gallbladder disease
Risk of diabetic retinopathy complications in patients with diabetes
Thyroid C-cell tumours (contraindicated in patients with personal/family history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2)
There is no official link established between Wegovy use and increased mortality in older adults, though long-term safety data specific to seniors remains limited. Post-marketing surveillance continues to monitor safety outcomes across all age groups. Patients and healthcare professionals are encouraged to report suspected adverse reactions via the MHRA Yellow Card Scheme.
Prescribing Wegovy to older adults requires careful consideration of age-related physiological changes and concurrent health conditions. Renal function naturally declines with age, and whilst semaglutide does not require dose adjustment for mild to moderate renal impairment, severe renal impairment or end-stage renal disease warrants caution due to limited clinical experience in these populations.
Sarcopenia and frailty represent significant concerns when initiating weight loss therapy in seniors. Rapid or excessive weight loss can accelerate muscle mass depletion, particularly if not accompanied by adequate protein intake and resistance exercise. Older adults losing weight should be monitored for signs of functional decline, reduced grip strength, or decreased mobility. NICE guidance emphasises that weight management interventions should preserve or enhance functional capacity rather than compromise it.
Polypharmacy considerations: Many older adults take multiple medications, increasing the potential for drug interactions. Wegovy delays gastric emptying, which theoretically may affect the absorption of oral medications, particularly those requiring rapid absorption or those with narrow therapeutic windows. According to the SmPC, no clinically relevant interactions have been identified, but caution is advised with:
Insulin and sulfonylureas (increased hypoglycaemia risk)
Warfarin (theoretical potential for altered absorption; consider more frequent INR monitoring initially)
Levothyroxine (theoretical interaction; monitor thyroid function if clinically indicated)
Medications requiring rapid gastrointestinal absorption
Cognitive function and self-management: Successful use of Wegovy requires patients to self-administer weekly injections, recognise and report adverse effects, and adhere to dietary modifications. Older adults with cognitive impairment, visual difficulties, or reduced manual dexterity may require additional support or may not be suitable candidates. Assessment of the patient's ability to manage the treatment regimen independently or with caregiver support is essential before initiation.
Wegovy follows a standardised dose escalation schedule designed to minimise gastrointestinal side effects, starting at 0.25 mg weekly and increasing monthly to the maintenance dose of 2.4 mg weekly over 16-20 weeks. For older adults, this gradual titration remains appropriate, though some clinicians may consider slower escalation if tolerability issues arise.
Baseline assessments before initiating Wegovy in seniors should include:
Comprehensive medication review
Renal function tests (eGFR, creatinine)
Liver function tests
Thyroid function (TSH) if clinically indicated
HbA1c for patients with diabetes
Nutritional status assessment
Frailty screening using validated tools (e.g., Clinical Frailty Scale)
Assessment of functional capacity and mobility
Ongoing monitoring is particularly important for older patients. Monthly reviews during dose escalation allow early identification of adverse effects and assessment of tolerability. Key monitoring parameters include:
Weight and body composition: Regular monitoring to ensure weight loss is not excessive (>1-2 kg per week) and to detect concerning muscle loss
Nutritional intake: Ensuring adequate protein (1.0-1.2 g/kg body weight daily) and micronutrient consumption
Hydration status: Particularly important given gastrointestinal side effects
Blood glucose: For patients with diabetes, more frequent monitoring may be needed, with potential reduction in insulin or sulfonylureas to prevent hypoglycaemia
Functional assessments: Regular evaluation of mobility, strength, and activities of daily living
Dose adjustments: If significant gastrointestinal side effects occur, delaying dose escalation or temporarily reducing the dose may be appropriate. Unlike younger patients, there may be less urgency to reach the maximum dose if lower doses provide adequate benefit with better tolerability. The treatment goal should prioritise overall health and function rather than maximum weight loss.
NICE stopping criteria: Treatment should be discontinued if a patient has not lost at least 5% of their initial body weight after 6 months at the maintenance dose of 2.4 mg weekly. Treatment is also limited to a maximum of 2 years.
Certain clinical scenarios make Wegovy inappropriate or require extreme caution in older adults. Absolute contraindications apply regardless of age and include:
Personal or family history of medullary thyroid carcinoma
Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)
Hypersensitivity to semaglutide or any excipients
Pregnancy or breastfeeding
Specific concerns for seniors that may preclude Wegovy use include:
Advanced frailty: Patients with severe frailty (Clinical Frailty Scale score ≥7) are unlikely to benefit from weight loss and may experience accelerated functional decline. In very frail older adults, maintaining adequate nutrition often takes priority over weight reduction.
Significant sarcopenia: Older adults with pre-existing severe muscle depletion should not undergo further weight loss without specialist input. Dual-energy X-ray absorptiometry (DEXA) scanning may be considered to assess body composition before treatment initiation in at-risk individuals.
Severe gastrointestinal disease: Conditions such as inflammatory bowel disease, severe gastroparesis, or history of bowel obstruction may be exacerbated by Wegovy's effects on gastrointestinal motility.
Limited life expectancy: For patients with advanced terminal illness or very limited life expectancy, the risks and burden of treatment may outweigh potential benefits.
Inadequate support systems: Older adults who cannot safely self-administer injections and lack reliable caregiver support may not be suitable candidates.
History of pancreatitis: A history of pancreatitis is not an absolute contraindication but requires caution. Wegovy should be discontinued if pancreatitis is suspected during treatment. Patients should be advised to seek immediate medical attention if they experience severe, persistent abdominal pain.
If you are an older adult considering Wegovy for weight management, an open and thorough discussion with your GP or specialist is essential. Prepare for your appointment by:
Listing all current medications, including over-the-counter products and supplements
Documenting your weight history and previous weight loss attempts
Noting any relevant medical conditions, particularly diabetes, heart disease, kidney problems, or gastrointestinal disorders
Considering your personal health goals and what you hope to achieve through weight loss
Questions to ask your healthcare provider:
What are the potential benefits of Wegovy specifically for my age and health status?
How will my other medications interact with Wegovy?
What monitoring will be required, and how frequently?
What dietary and exercise modifications should accompany treatment?
How will we ensure I maintain muscle mass during weight loss?
What side effects should prompt me to contact you urgently?
Are there alternative weight management approaches more suitable for my circumstances?
When to contact your GP urgently whilst taking Wegovy:
Severe, persistent abdominal pain (possible pancreatitis)
Persistent vomiting preventing adequate fluid intake
Signs of dehydration (dizziness, reduced urination, confusion)
Symptoms of hypoglycaemia if you have diabetes (shakiness, sweating, confusion)
Unexplained rapid heartbeat or chest pain
Significant functional decline or weakness
Visual changes or deterioration (especially in patients with diabetes)
Severe right-upper-quadrant pain (possible gallbladder disease)
Shared decision-making is particularly important for older adults, where treatment decisions should align with individual values, life goals, and overall health priorities. For some seniors, modest weight loss with preserved function and quality of life may be more valuable than maximal weight reduction. Your healthcare provider should work with you to develop a personalised treatment plan that considers your unique circumstances, preferences, and health objectives, ensuring that Wegovy—if prescribed—forms part of a comprehensive, multidisciplinary approach to healthy ageing.
Remember that suspected side effects can be reported via the MHRA Yellow Card Scheme at yellowcard.mhra.gov.uk or by searching for 'MHRA Yellow Card' in the Google Play or Apple App Store.
Adults over 65 can safely use Wegovy when appropriately selected and monitored, though individualised assessment is essential. Clinical trials show comparable efficacy and side effects in older adults, but careful evaluation of frailty status, sarcopenia risk, renal function, and polypharmacy is required before initiation.
Key safety concerns include gastrointestinal side effects leading to dehydration, accelerated muscle loss (sarcopenia), medication interactions particularly with diabetes treatments, and functional decline in frail individuals. Regular monitoring of nutritional status, hydration, and functional capacity is essential throughout treatment.
Wegovy is unsuitable for seniors with advanced frailty, severe pre-existing sarcopenia, personal or family history of medullary thyroid carcinoma, MEN 2 syndrome, or those unable to self-administer injections without adequate support. Patients with severe gastrointestinal disease or limited life expectancy should also avoid treatment.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.