
Prednisolone and Wegovy are two medications with distinctly different therapeutic purposes that some patients may need to take concurrently. Prednisolone is a corticosteroid used to treat inflammatory and autoimmune conditions, whilst Wegovy (semaglutide 2.4 mg) is a GLP-1 receptor agonist licensed for chronic weight management in specific patient groups. Although there is no absolute contraindication to using these medications together, their opposing metabolic effects—particularly on weight and blood glucose—require careful clinical consideration. This article examines the safety, interactions, and monitoring requirements when prednisolone and Wegovy are prescribed simultaneously, providing evidence-based guidance for patients and healthcare professionals in the UK.
Quick Answer: Prednisolone and Wegovy can be taken together as there is no absolute contraindication, but their opposing metabolic effects on weight and blood glucose require careful monitoring and individualised clinical assessment.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HerePrednisolone is a synthetic corticosteroid medication commonly prescribed in the UK for its potent anti-inflammatory and immunosuppressive properties. It works by mimicking cortisol, a naturally occurring hormone produced by the adrenal glands, and is used to treat a diverse range of conditions including rheumatoid arthritis, inflammatory bowel disease, asthma exacerbations, and autoimmune disorders. Prednisolone suppresses the immune system and reduces inflammation by inhibiting the production of inflammatory mediators such as prostaglandins and cytokines. The medication is typically prescribed in varying doses depending on the condition being treated, with treatment duration ranging from short courses for acute flare-ups to long-term maintenance therapy for chronic conditions.
Wegovy (semaglutide 2.4 mg) is a glucagon-like peptide-1 (GLP-1) receptor agonist licensed by the MHRA for chronic weight management in adults with obesity (BMI ≥30 kg/m²) or overweight (BMI ≥27 kg/m²) with at least one weight-related comorbidity. However, NHS access is restricted by NICE Technology Appraisal 875 to specific patient cohorts. Administered as a once-weekly subcutaneous injection, Wegovy follows a dose escalation schedule (starting at 0.25 mg and gradually increasing to the 2.4 mg maintenance dose) to improve tolerability. It works by mimicking the action of the naturally occurring hormone GLP-1, which regulates appetite and food intake. The medication slows gastric emptying, increases feelings of satiety, and reduces hunger signals in the brain, thereby facilitating weight loss when combined with a reduced-calorie diet and increased physical activity.
Both medications serve distinctly different therapeutic purposes and act through entirely separate physiological mechanisms. Understanding how each drug functions is essential when considering their concurrent use, particularly given prednisolone's metabolic effects and Wegovy's role in weight management and glycaemic control. Notably, Wegovy is contraindicated in pregnancy and requires caution in patients with a history of pancreatitis or gallbladder disease.
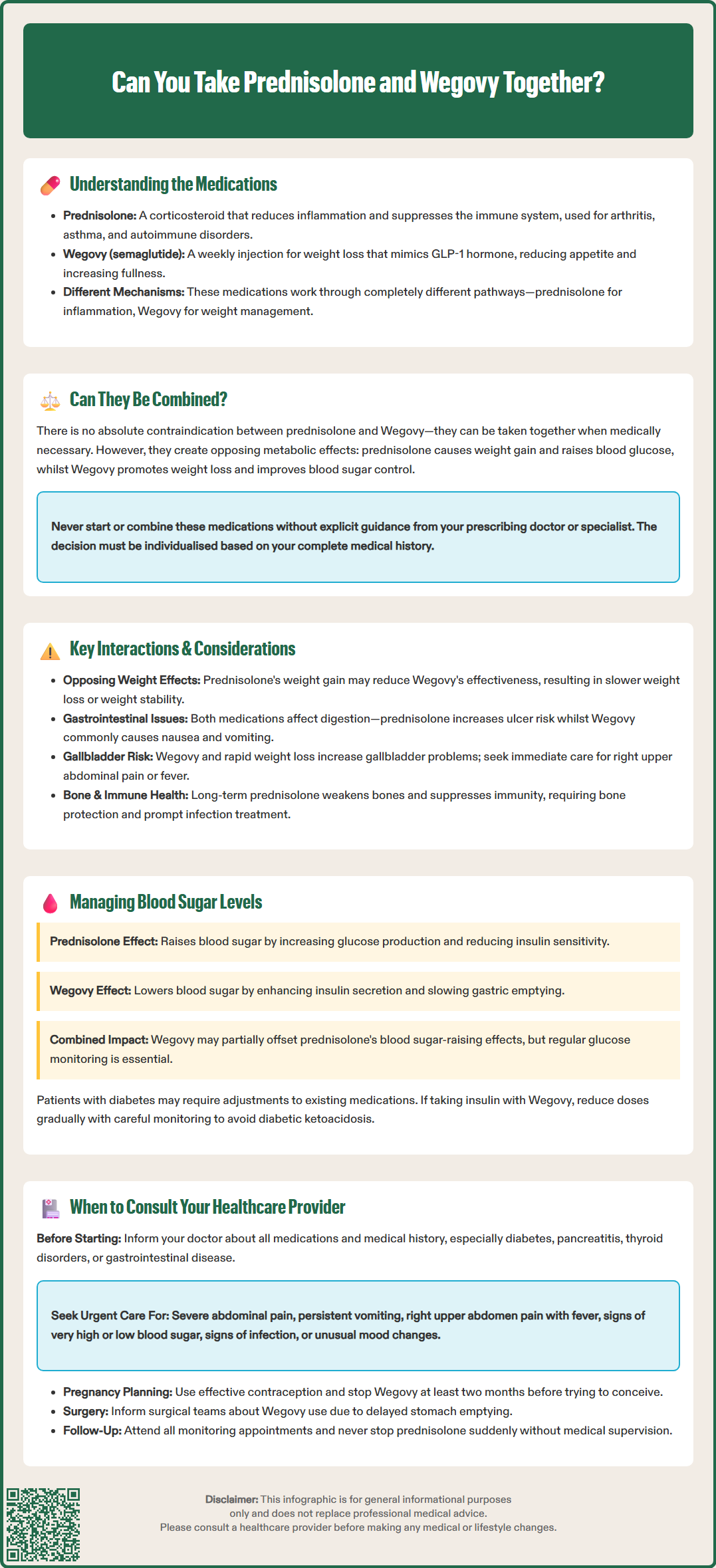
There is no absolute contraindication to taking prednisolone and Wegovy concurrently, and no direct pharmacokinetic interaction between these two medications has been officially documented in the Summary of Product Characteristics (SmPC) for either drug. While Wegovy does delay gastric emptying, this effect has not shown clinically relevant impacts on the absorption of most oral medicines according to the SmPC. Many patients may require both medications simultaneously—for instance, someone with rheumatoid arthritis requiring corticosteroid therapy who also has obesity and associated metabolic complications.
However, whilst there is no official prohibition against combining these medications, healthcare professionals must carefully consider the indirect metabolic effects that may arise from their concurrent use. The decision to prescribe both medications together should be made on an individual basis, taking into account the patient's complete medical history, current health status, and the specific indications for each treatment.
It is important to note that prednisolone and Wegovy have opposing effects on certain metabolic parameters, particularly glucose metabolism and weight. Prednisolone commonly causes weight gain and can elevate blood glucose levels, whilst Wegovy is specifically designed to promote weight loss and has beneficial effects on glycaemic control. This creates a complex clinical scenario that requires careful monitoring and management.
Patients should never start, stop, or combine these medications without explicit guidance from their prescribing clinician. It's worth noting that in the UK, Wegovy initiation typically occurs in specialist weight management services under NICE TA875. A thorough discussion with your GP or specialist is essential to ensure that the benefits of concurrent therapy outweigh any potential risks, and that appropriate monitoring arrangements are in place.
Whilst prednisolone and Wegovy do not interact directly at a pharmacological level, several important clinical considerations emerge when these medications are used together, primarily relating to their contrasting metabolic effects.
Opposing effects on body weight: Prednisolone is well-known for causing weight gain through multiple mechanisms, including increased appetite, fluid retention, and redistribution of body fat (particularly to the face, neck, and trunk). Conversely, Wegovy is prescribed specifically to achieve weight loss. When used concurrently, prednisolone may partially counteract Wegovy's weight-reducing effects, potentially diminishing the overall efficacy of weight management therapy. Patients may experience slower weight loss than anticipated or, in some cases, weight stability rather than reduction.
Gastrointestinal considerations: Both medications can affect the gastrointestinal system, though through different mechanisms. Prednisolone may increase the risk of peptic ulceration and gastritis, particularly when used long-term, at higher doses, or concurrently with NSAIDs. Gastroprotection should be considered in these higher-risk scenarios according to NICE Clinical Knowledge Summaries guidance. Wegovy commonly causes gastrointestinal adverse effects including nausea, vomiting, diarrhoea, and constipation, especially during dose escalation. The combination may potentially increase the overall burden of gastrointestinal symptoms, though there is no evidence of a synergistic effect.
Gallbladder disease: Wegovy and rapid weight loss are both associated with an increased risk of gallbladder disorders including cholelithiasis and cholecystitis. Patients should be advised to seek prompt medical attention if they experience symptoms such as right upper quadrant pain, fever, or jaundice.
Immune function: Prednisolone suppresses immune function, which is often its intended therapeutic effect. Patients taking immunosuppressive doses of corticosteroids should avoid live vaccines and be aware that any concurrent illness or infection may require prompt medical attention. Whilst Wegovy does not affect immune function, patients experiencing significant gastrointestinal side effects may become dehydrated, which could complicate management of other conditions.
Bone health: Long-term systemic corticosteroid use increases the risk of osteoporosis. Assessment for bone protection measures should be considered according to UK guidance for patients on prolonged prednisolone therapy.
Cardiovascular considerations: Long-term corticosteroid use can contribute to hypertension and cardiovascular risk, whilst weight loss achieved with Wegovy may help reduce these same risks. Careful cardiovascular monitoring is advisable when both medications are used together.
Diabetic retinopathy: In people with diabetes, rapid improvements in glycaemic control with GLP-1 receptor agonists may be associated with temporary worsening of diabetic retinopathy. Appropriate ophthalmological monitoring should be considered.
One of the most clinically significant considerations when combining prednisolone and Wegovy relates to glucose metabolism and diabetes risk. These medications have substantially different—and in some respects opposing—effects on blood sugar control.
Prednisolone's effects on glucose: Corticosteroids like prednisolone are well-established causes of hyperglycaemia (elevated blood glucose). Prednisolone increases blood sugar through several mechanisms: it promotes gluconeogenesis (glucose production) in the liver, reduces insulin sensitivity in peripheral tissues, and can impair insulin secretion from pancreatic beta cells. This can lead to steroid-induced hyperglycaemia in people without diabetes, or significantly worsen glycaemic control in those with pre-existing type 2 diabetes. The effect is dose-dependent and typically more pronounced with higher doses and longer treatment duration.
Wegovy's beneficial effects on glucose: In contrast, GLP-1 receptor agonists like Wegovy have favourable effects on glucose metabolism. Semaglutide enhances glucose-dependent insulin secretion, suppresses inappropriately elevated glucagon secretion, and slows gastric emptying. These mechanisms contribute to improved glycaemic control, and clinical trials have demonstrated that Wegovy can reduce HbA1c levels in people with type 2 diabetes.
Clinical implications: When used together, Wegovy's glucose-lowering effects may help partially mitigate prednisolone's tendency to raise blood sugar levels. However, this should not be relied upon as a primary strategy for managing steroid-induced hyperglycaemia. Patients with diabetes or at risk of diabetes who require both medications should undergo regular blood glucose monitoring. For those with established diabetes, adjustments to existing diabetes medications (such as metformin, sulfonylureas, or insulin) may be necessary.
Insulin adjustment caution: The MHRA has issued a Drug Safety Update warning about the risk of diabetic ketoacidosis when insulin is rapidly reduced upon starting GLP-1 receptor agonists. Insulin doses should be reduced gradually and with careful monitoring when initiating Wegovy in insulin-treated patients.
Monitoring recommendations: NICE Clinical Knowledge Summaries on oral corticosteroids advise that patients on long-term therapy should be monitored for the development of diabetes. The Joint British Diabetes Societies (JBDS) also provide guidance on managing steroid-induced hyperglycaemia. When Wegovy is added to a corticosteroid regimen, or vice versa, baseline and periodic monitoring of fasting glucose or HbA1c is advisable. Patients should be educated about symptoms of hyperglycaemia (increased thirst, frequent urination, fatigue) and hypoglycaemia if taking other diabetes medications.
Patients taking prednisolone and Wegovy concurrently should maintain regular contact with their healthcare team and be aware of specific circumstances that warrant prompt medical consultation.
Before starting combined therapy: Always inform your GP or specialist about all medications you are currently taking before starting either prednisolone or Wegovy. Discuss your complete medical history, including any history of diabetes, pancreatitis, thyroid disorders, or gastrointestinal disease. Your healthcare provider can assess whether concurrent use is appropriate for your individual circumstances and establish a suitable monitoring plan.
Seek urgent medical advice if you experience:
Severe or persistent gastrointestinal symptoms: Whilst mild nausea is common with Wegovy, severe or persistent vomiting, severe abdominal pain (especially if radiating to the back), or signs of dehydration require immediate assessment, as these may indicate pancreatitis or other serious complications.
Gallbladder symptoms: Right upper quadrant pain, fever, or jaundice may indicate gallbladder disease, which is associated with GLP-1 receptor agonists and rapid weight loss.
Signs of hyperglycaemia: Excessive thirst, frequent urination, unexplained fatigue, blurred vision, or slow-healing wounds may indicate poorly controlled blood sugar and require medication adjustment.
Symptoms of hypoglycaemia: If you take other diabetes medications alongside Wegovy, be alert for symptoms of low blood sugar including trembling, sweating, confusion, or palpitations.
Unusual mood changes or psychological symptoms: Corticosteroids can affect mood and mental health; report any significant changes in mood, sleep disturbance, or psychological symptoms.
Signs of infection: Because prednisolone suppresses immune function, any fever, persistent cough, or other signs of infection should be evaluated promptly.
Important additional considerations:
Pregnancy planning: Wegovy is contraindicated in pregnancy. Women of childbearing potential should use effective contraception during treatment and inform their healthcare provider immediately if pregnancy occurs or is planned. Wegovy should be discontinued at least two months before a planned pregnancy according to the SmPC.
Surgical procedures: Inform your surgical and anaesthetic teams about GLP-1 receptor agonist use before any planned procedures, as these medications may affect gastric emptying and potentially increase aspiration risk. Follow local perioperative guidance.
Vaccinations: Avoid live vaccines during high-dose systemic corticosteroid therapy. Check with your healthcare provider before receiving any vaccinations.
Routine monitoring: Attend all scheduled follow-up appointments for monitoring of weight, blood pressure, blood glucose, and assessment of treatment efficacy and adverse effects. Your healthcare provider may recommend periodic blood tests to monitor metabolic parameters. Never discontinue prednisolone abruptly without medical guidance, as this can cause adrenal insufficiency. Similarly, discuss any concerns about Wegovy with your prescriber before stopping treatment, as gradual dose adjustments may be more appropriate than sudden cessation.
Yes, there is no absolute contraindication to taking prednisolone and Wegovy together, and no direct drug interaction has been documented. However, their opposing effects on weight and blood glucose require individualised assessment and careful monitoring by your healthcare provider.
Prednisolone commonly causes weight gain through increased appetite and fluid retention, which may counteract Wegovy's weight-reducing effects. Patients may experience slower weight loss than expected or weight stability rather than reduction when taking both medications concurrently.
Yes, regular blood glucose monitoring is advisable when taking both medications, as prednisolone raises blood sugar whilst Wegovy lowers it. Patients with diabetes or at risk of diabetes should have baseline and periodic monitoring of fasting glucose or HbA1c, with potential adjustments to diabetes medications.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.