
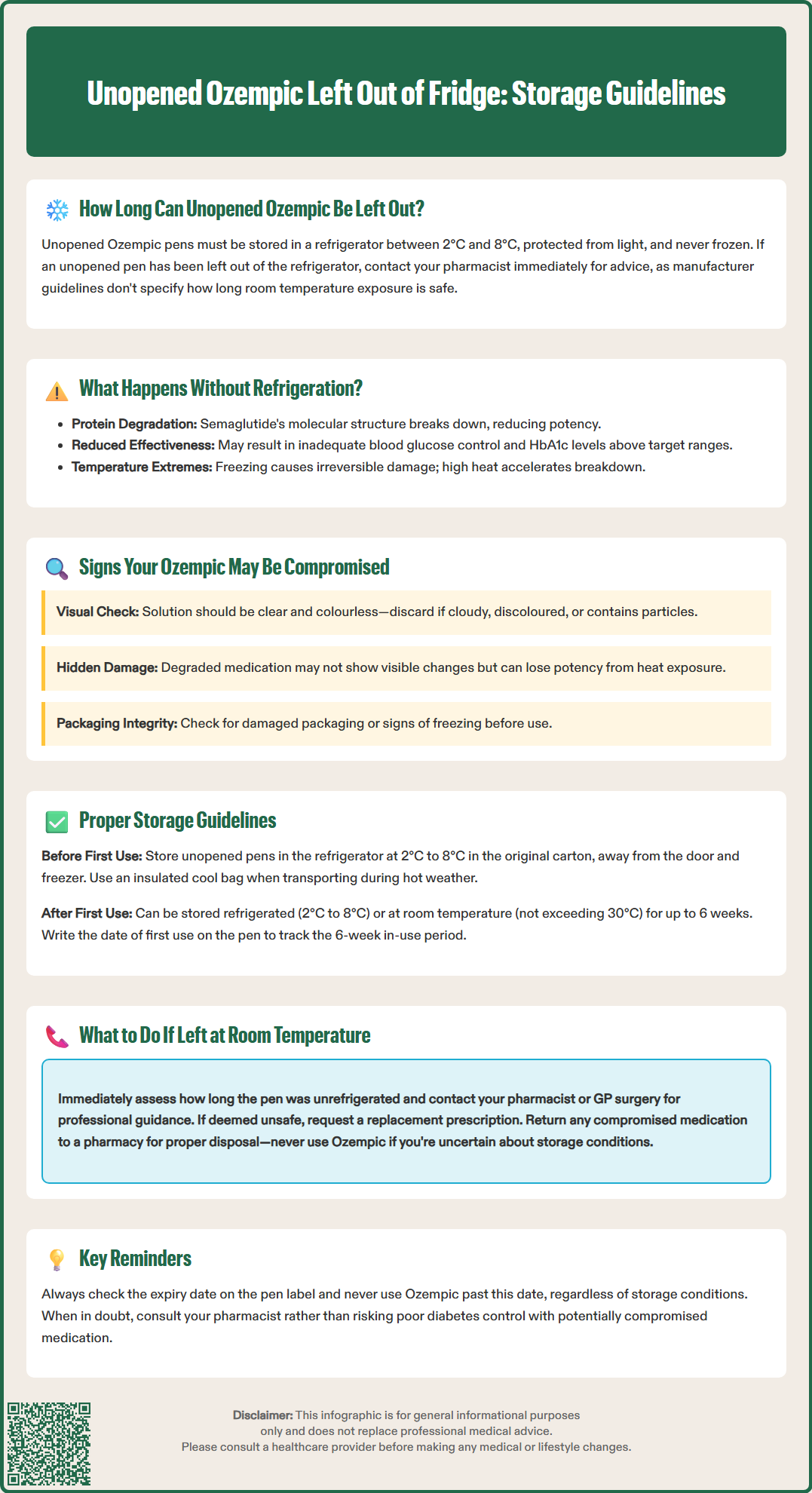
Unopened Ozempic left out of fridge requires careful assessment before use. Unopened Ozempic (semaglutide) pens must be stored refrigerated between 2°C and 8°C until first use, as specified in UK product information. If your unopened pen has been left unrefrigerated, this represents a deviation from recommended storage conditions that may compromise the medication's stability and efficacy. The UK Summary of Product Characteristics does not specify an allowable duration for room temperature storage of unopened pens. Contact your pharmacist immediately for advice specific to your situation, as they can assess factors including duration of temperature exposure and whether the medication remains suitable for use.
Quick Answer: Unopened Ozempic pens must be stored refrigerated between 2°C and 8°C, and if left out of the fridge, you should contact your pharmacist immediately for advice as the medication's stability may be compromised.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereUnopened Ozempic (semaglutide) pens should be stored in a refrigerator between 2°C and 8°C until first use, as specified in the manufacturer's storage instructions. According to the UK product information, unopened pens must be kept refrigerated, protected from light, and must not be frozen.
If your unopened Ozempic pen has been left out of the refrigerator, this represents a deviation from the recommended storage conditions. The UK Summary of Product Characteristics (SmPC) and Patient Information Leaflet (PIL) do not specify a specific allowable duration for room temperature storage of unopened pens.
If you discover that your unopened Ozempic has been left unrefrigerated, you should contact your pharmacist for advice specific to your situation. They can assess factors such as the duration of the temperature excursion, the approximate temperature to which the pen was exposed, and whether the medication remains suitable for use.
It's important to note that the stability of semaglutide, the active ingredient in Ozempic, can be compromised by exposure to temperatures outside the recommended range, potentially affecting its efficacy and safety profile.
Patients should always check the expiry date printed on the pen label and carton. Even if stored correctly, Ozempic should never be used beyond its expiry date. If you are uncertain about how long your unopened pen has been out of the fridge, or if it has been exposed to high temperatures, contact your pharmacist or GP surgery for advice before using it.
Semaglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist, a peptide-based medication that requires careful temperature control to maintain its molecular structure and therapeutic activity. When unopened Ozempic is stored outside the recommended refrigerated conditions, several potential issues may arise, though the severity depends on the duration and degree of temperature exposure.
Protein degradation is the primary concern with improper storage of peptide medications. Semaglutide's complex molecular structure can begin to break down when exposed to heat, light, or temperature fluctuations. This degradation may not be visible to the naked eye but can potentially reduce the medication's potency. If you use Ozempic that has been improperly stored, you may not achieve adequate blood glucose control, potentially leading to hyperglycaemia and suboptimal management of your type 2 diabetes.
Using medication with potentially reduced efficacy means you may not receive the intended therapeutic benefit, including the glucose-lowering effects. For patients prescribed Ozempic, this could result in poor glycaemic control, with HbA1c levels remaining above individualised target ranges. NICE guidance (NG28) recommends that HbA1c targets should be personalised based on factors including the person's needs and circumstances, with a general target of 48-53 mmol/mol (6.5-7.0%) for many adults with type 2 diabetes if it can be achieved safely.
Temperature extremes pose additional risks. Exposure to freezing temperatures can cause irreversible damage to the medication, whilst high temperatures accelerate degradation. Neither frozen nor overheated Ozempic should ever be used, regardless of whether the pen remains unopened. If you suspect your medication has been exposed to extreme temperatures, you should seek advice from a healthcare professional before use.
Visual inspection is the first step in determining whether your unopened Ozempic pen may have been compromised by improper storage. According to the UK Patient Information Leaflet, Ozempic solution should be clear and colourless. Before using any pen, hold it up to the light and examine the liquid carefully through the viewing window.
Key warning signs that indicate the medication should not be used include:
Cloudiness or turbidity – the solution should be completely clear, not milky or hazy
Visible particles or precipitates – any floating matter, crystals, or sediment indicates degradation
Discolouration – any unusual colour changes
Frozen medication – if the pen has been frozen, even if subsequently thawed, it must be discarded
Damaged packaging – cracks, leaks, or compromised seals on the pen or carton
It is important to understand that not all degraded medication will show visible changes. Semaglutide can potentially lose potency without any apparent alteration in appearance, particularly if it has been stored at elevated temperatures for extended periods. This is why adhering to storage instructions is crucial, even when the solution looks normal.
If you have any doubt about your medication's integrity, do not use it. Contact your community pharmacist, who can inspect the pen and provide guidance. Your pharmacist can also liaise with your GP surgery regarding a replacement prescription if necessary. Never attempt to use Ozempic that you suspect may have been improperly stored, as this could lead to inadequate diabetes management.
If you need to dispose of unused medication, return it to a pharmacy for safe disposal. Never dispose of medicines in household waste or down the drain.
Correct storage of unopened Ozempic is essential to maintain the medication's stability and ensure you receive its full therapeutic benefit. The UK Summary of Product Characteristics (SmPC) and Patient Information Leaflet (PIL) provide clear instructions that should be followed carefully from the moment you collect your prescription.
Refrigerated storage (2°C to 8°C) is the required condition for unopened Ozempic pens. Store the pen in its original carton to protect it from light, and place it in the main body of your refrigerator – not in the door, where temperature fluctuations are more common, and never in the freezer compartment. The medication should be kept away from the freezer element to prevent accidental freezing. Many patients find it helpful to designate a specific area of the refrigerator for medications to avoid confusion with food items.
When transporting Ozempic from the pharmacy, consider using an insulated cool bag during hot weather or for longer journeys. Most pharmacies can provide advice on safe transport. If you are travelling and need to carry Ozempic, discuss appropriate storage options with your pharmacist before your journey.
Additional storage considerations include:
Keep Ozempic out of direct sunlight and away from heat sources
Store in a secure location away from children and pets
Do not remove the pen cap until you are ready for first use
Check the expiry date regularly and rotate stock if you have multiple pens
Maintain a storage log if you are concerned about tracking temperature exposure
Once you have used an Ozempic pen for the first time, storage requirements change. After first use, according to the UK SmPC, the pen may be stored either in the refrigerator (2°C to 8°C) or at room temperature (not exceeding 30°C) for up to 6 weeks (42 days). It is advisable to write the date of first use on the pen to track the 6-week in-use period.
If you discover that your unopened Ozempic has been left out of the fridge, do not panic – but do take immediate action to assess the situation and determine whether the medication remains safe to use. Your response should be guided by how long the pen has been at room temperature and the conditions to which it has been exposed.
First, establish the timeline. Consider how long the pen has been unrefrigerated and whether it has been exposed to extreme heat, direct sunlight, or freezing conditions. Make a note of when the pen was removed from refrigeration and the approximate temperature conditions.
Contact your pharmacist or GP surgery for advice. They can provide guidance based on the specific circumstances of your storage deviation. The pharmacist may consult the manufacturer's stability data or contact Novo Nordisk directly to determine if the medication remains suitable for use.
If the storage conditions are deemed unacceptable, you will need to request a replacement prescription from your GP. Explain the circumstances clearly. Be aware that you may need to pay the standard NHS prescription charge (unless you are exempt or have a prescription prepayment certificate) for the replacement medication, as there is no automatic entitlement to free replacements for storage issues.
For temperature extremes or uncertain conditions, err on the side of caution. If the pen has been exposed to high temperatures (such as being left in a car during summer), frozen, or subjected to unknown storage conditions during postal delivery, seek professional advice before use. Perform a visual inspection as described earlier, but remember that the absence of visible changes does not guarantee the medication's potency.
Document the incident for your records, particularly if the storage failure occurred during pharmacy delivery. Most pharmacies and delivery services have protocols for addressing medications damaged during transport. If you experience recurrent storage issues, discuss alternative arrangements with your healthcare team, such as smaller prescription quantities or more frequent collections.
For safe disposal of any compromised medication, return it to a pharmacy. Do not dispose of medicines in household waste or down the drain.
Contact your pharmacist immediately for advice, as UK product information does not specify an allowable duration for room temperature storage of unopened pens. The pharmacist can assess the specific circumstances and determine if the medication remains suitable for use.
Check for cloudiness, visible particles, discolouration, or signs of freezing. However, degradation may occur without visible changes, so always seek professional advice if storage conditions have been compromised.
Do not use the medication until you have consulted your pharmacist or GP surgery. If deemed unsuitable for use, return it to a pharmacy for safe disposal and request a replacement prescription from your GP.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.