
Should Ozempic be refrigerated? This is a crucial question for anyone prescribed this type 2 diabetes medication. Ozempic (semaglutide) is a biological medicine requiring specific storage conditions to maintain its effectiveness and safety. Unopened pens must be refrigerated between 2°C and 8°C, whilst opened pens may be stored at room temperature up to 30°C for six weeks. Understanding these requirements ensures your medication remains stable and therapeutically effective. This article provides comprehensive guidance on refrigeration, storage after opening, travel considerations, and what to do if storage guidelines are not followed, aligned with MHRA and manufacturer recommendations.
Quick Answer: Unopened Ozempic must be refrigerated between 2°C and 8°C, whilst opened pens may be stored refrigerated or at room temperature up to 30°C for six weeks.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereOzempic (semaglutide) is a glucagon-like peptide-1 (GLP-1) receptor agonist prescribed for adults with type 2 diabetes mellitus. As a biological medication, proper storage is essential to maintain its stability, efficacy, and safety. The Medicines and Healthcare products Regulatory Agency (MHRA) and manufacturer guidelines provide clear instructions on refrigeration requirements.
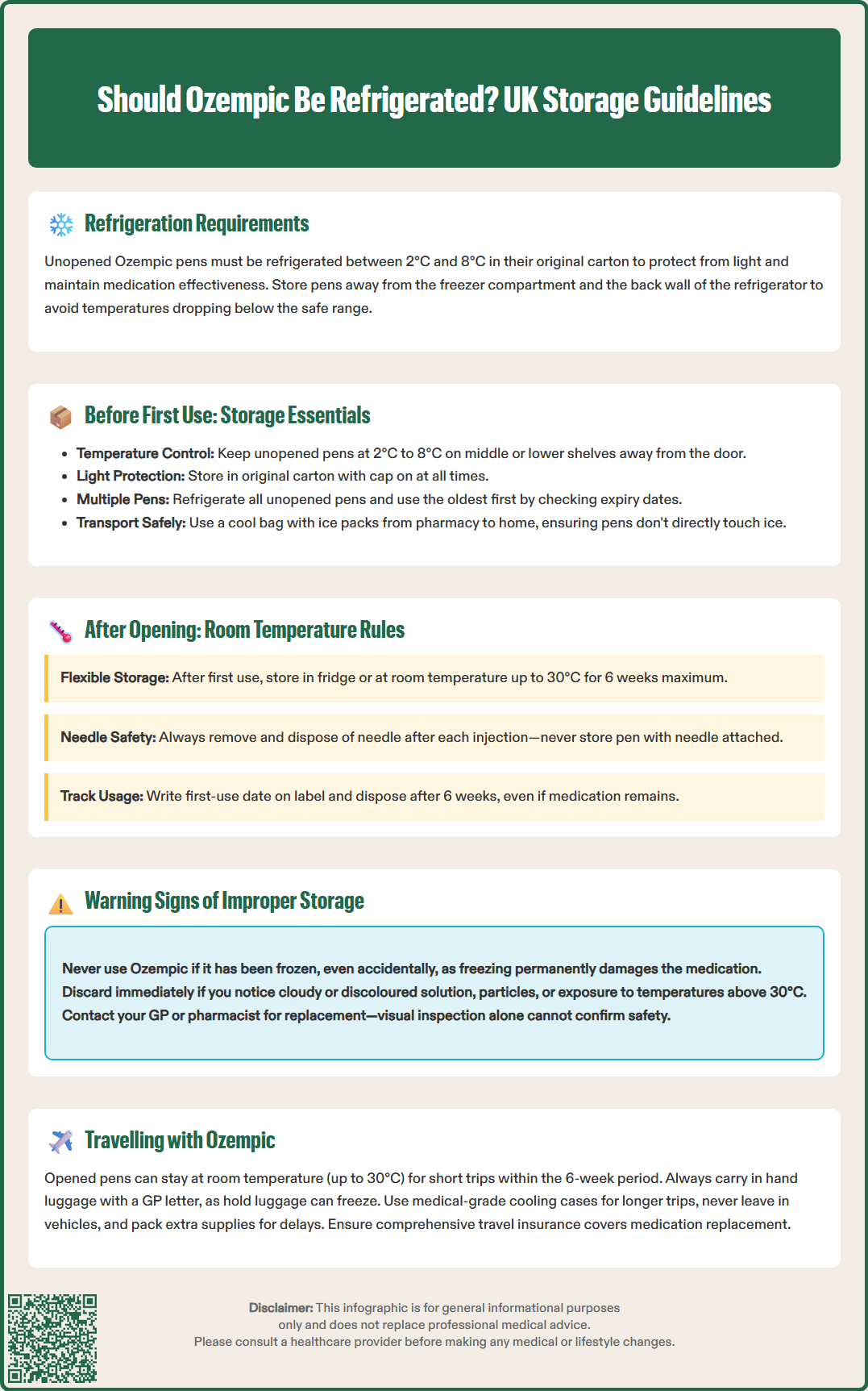
Unopened Ozempic pens must be stored in a refrigerator between 2°C and 8°C until first use. This temperature range preserves the molecular structure of semaglutide, preventing degradation that could reduce therapeutic effectiveness. The pen should be kept in its original carton with the cap on to protect it from light, which can also affect the medication's stability.
It is crucial to avoid freezing Ozempic. If the medication has been frozen, even accidentally, it must not be used and should be safely disposed of according to local pharmacy guidelines. Freezing can irreversibly damage the protein structure of semaglutide, rendering it ineffective or potentially harmful.
Patients should store unopened pens away from the freezer compartment and avoid placing them directly against the back wall of the refrigerator, where temperatures may drop below the recommended range. Keep Ozempic out of sight and reach of children. Understanding these fundamental storage requirements helps ensure that your medication remains effective throughout its shelf life, which is stated on the packaging and typically extends to the expiry date when stored correctly.
Importantly, never store Ozempic with a needle attached to the pen, as this can affect the sterility of the medication and may lead to leakage or inaccurate dosing.
Before you use your Ozempic pen for the first time, strict refrigeration between 2°C and 8°C is mandatory. This applies from the moment you collect your prescription from the pharmacy until you administer your first dose. Most household refrigerators maintain this temperature range in the main compartment, but it is advisable to use a refrigerator thermometer if you have concerns about temperature consistency.
Practical storage tips for unopened Ozempic include:
Store the pen in the middle or lower shelves of your refrigerator, where temperature is most stable
Keep the pen in its original carton to protect from light exposure
Avoid storing in the refrigerator door, where temperature fluctuations occur with frequent opening
Do not place near the freezer compartment or cooling elements
Always keep the pen cap on to protect the medication
If you receive multiple pens at once (for example, a three-month supply), all unopened pens should remain refrigerated until needed. Check the expiry date printed on each pen and carton, and use the oldest pen first to ensure you do not inadvertently keep medication beyond its expiry date.
When transporting Ozempic from the pharmacy to your home, minimise the time the medication is unrefrigerated. If you anticipate a longer journey, consider using a cool bag with ice packs, ensuring the pen does not come into direct contact with ice or frozen gel packs to prevent freezing. Never leave Ozempic in a hot car, as temperatures can quickly exceed safe limits, particularly during warmer months. If you're uncertain whether your unopened pen has been stored correctly during transport, consult your pharmacist for advice before using it.
Once you have used your Ozempic pen for the first time, the storage requirements change significantly. After the initial use, the pen may be stored either in the refrigerator (2°C to 8°C) or at room temperature, not exceeding 30°C, for up to 6 weeks (42 days). This flexibility makes daily management more convenient for most patients.
Many patients find room temperature storage more practical after opening, as it eliminates the need to handle a cold pen before each injection, which can be uncomfortable. The medication remains stable and effective at temperatures up to 30°C for the specified 6-week period. However, you must still protect the pen from direct sunlight and excessive heat sources such as radiators, windowsills, or hot vehicles.
Important considerations for in-use storage:
Always remove the needle after each injection and dispose of it safely
Replace the pen cap immediately after use to protect from light
Never store the pen with a needle attached
Store away from direct heat sources and sunlight
Keep out of reach of children and pets
Do not store in bathrooms, where humidity and temperature fluctuate
Write the date of first use on the pen label to track the 6-week limit
If you choose to continue refrigerating your opened pen, ensure you allow it to reach room temperature before injecting (approximately 15-30 minutes). Cold injections can be more uncomfortable and may increase injection site reactions. Never attempt to warm the pen artificially using hot water, microwaves, or other heat sources, as this will damage the medication.
After 6 weeks of first use, the pen must be disposed of, even if medication remains inside. This is because the stability of the medication can no longer be guaranteed beyond this period, regardless of storage conditions, according to the manufacturer's guidelines.
Improper storage of Ozempic can compromise the medication's effectiveness and safety. Temperature abuse—exposure to temperatures outside the recommended range—can cause irreversible changes to the semaglutide molecule, potentially reducing its therapeutic benefit or causing unpredictable effects.
If an unopened Ozempic pen has been left unrefrigerated, the UK product information does not specify a safe time limit at room temperature. If this occurs, you should contact your pharmacist for advice before using the medication. If the pen has been exposed to temperatures above 30°C or left unrefrigerated for an extended period, it should not be used. There is no reliable way to determine whether the medication has degraded simply by visual inspection, as semaglutide solutions typically remain clear and colourless even when compromised.
Freezing is particularly problematic. If Ozempic has been frozen, even briefly, the protein structure of semaglutide becomes denatured and cannot be restored. Frozen medication must be discarded immediately and should never be used, even if it appears normal after thawing. Using frozen medication could result in reduced blood glucose control or unpredictable effects.
Signs that Ozempic may have been improperly stored include:
The solution appears cloudy, discoloured, or contains particles
The pen has been frozen (even if subsequently thawed)
The pen has been exposed to direct sunlight for prolonged periods
Storage temperature has exceeded 30°C
If you suspect your Ozempic has been stored incorrectly, do not use it. Contact your GP surgery or pharmacist for guidance and to arrange a replacement prescription. Never attempt to use medication that may have been compromised, as this could affect your diabetes management and potentially cause harm. Your healthcare team can advise on interim blood glucose monitoring and management whilst awaiting replacement medication.
For disposal, return unused or compromised pens to your local pharmacy. Never dispose of medication in household waste. Used needles should be placed in a designated sharps bin, which can be obtained from your GP surgery or pharmacy, and disposed of according to local arrangements.
Travelling with Ozempic requires advance planning to ensure the medication remains within safe storage parameters throughout your journey. Whether travelling domestically within the UK or internationally, maintaining appropriate storage conditions is essential for medication efficacy and your health.
For short trips (day trips or overnight stays), an opened Ozempic pen can be carried at room temperature (up to 30°C) without refrigeration, provided it remains within the 6-week in-use period. Keep the pen in a cool, dark place such as an insulated medication pouch or the coolest part of your bag, away from direct sunlight.
For longer journeys or holidays, consider these practical strategies:
Use a medical-grade cooling case or insulated medication pouch designed for insulin and similar medications
Keep pens insulated but not in direct contact with ice/gel packs to avoid freezing
If staying in hotels, request refrigerator access in your room or ask reception to store medication in their refrigerator
For air travel, carry Ozempic in hand luggage (never check it in hold luggage, where temperatures can drop below freezing)
Carry a letter from your GP confirming your prescription and need for refrigerated medication
Pack extra supplies in case of travel delays or loss
Carry spare needles and a travel sharps container for safe disposal
Never store the pen with a needle attached
Air travel considerations are particularly important. Airport security regulations permit passengers to carry medical refrigeration packs and cooling cases. Inform security staff that you are carrying refrigerated medication. The pen should remain in its original packaging with the prescription label visible. Most airlines allow reasonable quantities of ice packs or gel packs for medical purposes, though these may need to be screened separately.
When travelling to warmer climates, never leave Ozempic in vehicles, even briefly, as internal temperatures can rapidly exceed 50°C in direct sunlight. If refrigeration is unavailable, store the medication in the coolest available location, such as a hotel room safe or the coolest cupboard, away from windows and heat sources. Monitor the ambient temperature where possible, and if conditions consistently exceed 30°C, seek refrigeration options or consult a local pharmacist about temporary storage solutions.
For international travel, research healthcare facilities at your destination in case you need emergency supplies or advice. The NHS provides guidance for travellers with diabetes, and many countries have reciprocal healthcare arrangements. Carry your Global Health Insurance Card (GHIC) or European Health Insurance Card (EHIC) where applicable. Always travel with comprehensive travel insurance that covers pre-existing conditions and medication replacement if needed.
If you experience any side effects from Ozempic, you can report them via the MHRA Yellow Card scheme at yellowcard.mhra.gov.uk or through the Yellow Card app.
Yes, once opened, Ozempic may be stored at room temperature up to 30°C or refrigerated between 2°C and 8°C for up to six weeks (42 days). After this period, the pen must be disposed of regardless of remaining medication.
If Ozempic has been frozen, even briefly, it must be discarded immediately and never used. Freezing irreversibly damages the semaglutide protein structure, rendering the medication ineffective or potentially harmful.
Carry Ozempic in hand luggage using an insulated medication pouch or cooling case, ensuring it does not freeze. Opened pens can remain at room temperature (up to 30°C) during travel within the six-week in-use period, whilst unopened pens require refrigeration between 2°C and 8°C.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.