
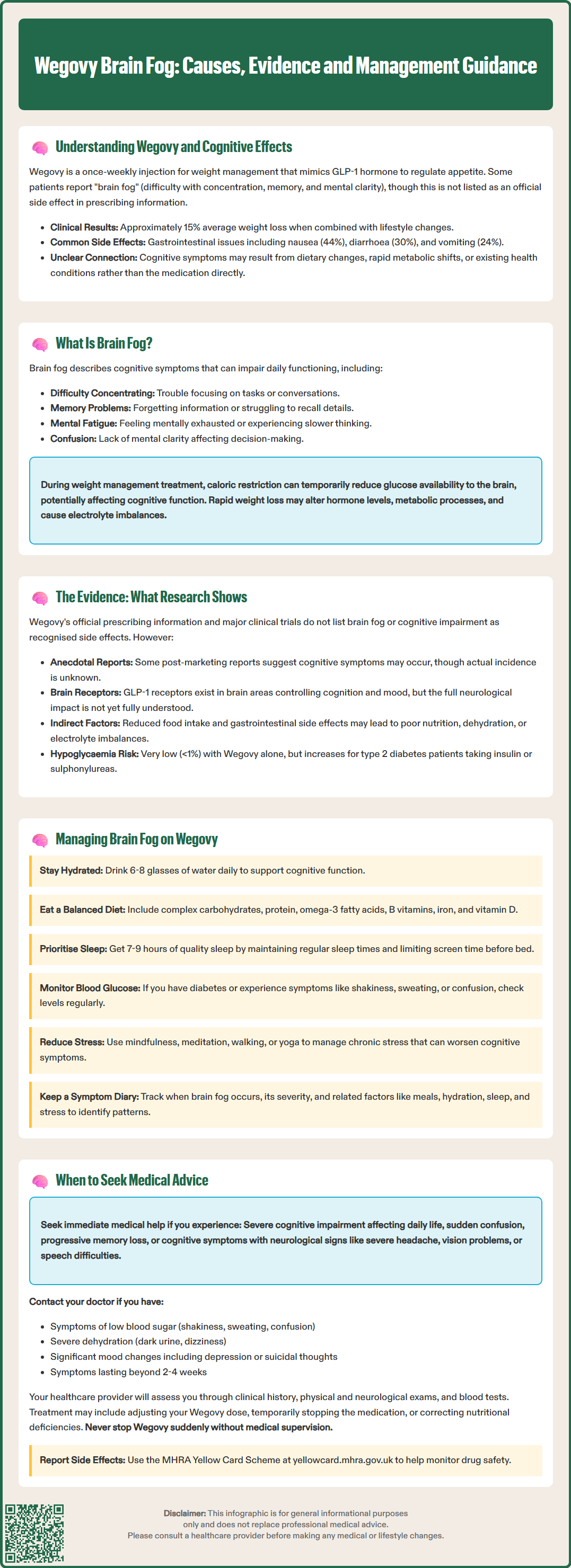
Wegovy (semaglutide 2.4 mg) is a GLP-1 receptor agonist licensed in the UK for weight management in adults with obesity or overweight with weight-related comorbidities. Whilst gastrointestinal side effects such as nausea and diarrhoea are well-documented, some individuals report experiencing 'brain fog'—cognitive symptoms including difficulty concentrating, memory problems, and reduced mental clarity. These symptoms are not listed as recognised adverse reactions in official prescribing information. Understanding whether cognitive changes are genuinely linked to Wegovy, or arise from dietary modifications, metabolic shifts, or other factors, is essential for patients and healthcare professionals managing weight loss treatment.
Quick Answer: Brain fog is not listed as an official adverse reaction to Wegovy in UK prescribing information, though some individuals report cognitive symptoms that may relate to dietary changes, dehydration, or metabolic factors rather than the medication itself.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereWegovy (semaglutide 2.4 mg) is a glucagon-like peptide-1 (GLP-1) receptor agonist licensed in the UK for weight management in adults with obesity or overweight with weight-related comorbidities. Administered as a once-weekly subcutaneous injection, Wegovy works by mimicking the naturally occurring hormone GLP-1, which regulates appetite and food intake through actions on the brain's satiety centres, particularly in the hypothalamus and brainstem.
Since receiving its marketing authorisation and subsequent recommendation by NICE (Technology Appraisal TA875, March 2023), Wegovy has demonstrated significant efficacy in clinical trials. In the pivotal STEP-1 trial, patients achieved average weight loss of approximately 15% from baseline when combined with lifestyle interventions. However, as with any medication, patients may experience various side effects during treatment.
Some individuals taking Wegovy have reported experiencing cognitive symptoms, colloquially termed 'brain fog'—a non-specific descriptor encompassing difficulties with concentration, memory, mental clarity, and processing speed. Whilst gastrointestinal adverse effects such as nausea (44%), diarrhoea (30%), and vomiting (24%) are well-documented in the Summary of Product Characteristics (SmPC), cognitive symptoms are not listed as recognised adverse reactions in official prescribing information.
Understanding whether there is a genuine pharmacological link between Wegovy and cognitive changes, or whether such symptoms arise from other factors associated with weight loss treatment (such as dietary changes, rapid metabolic shifts, or concurrent health conditions), is essential for both patients and healthcare professionals. This article examines the evidence surrounding Wegovy and brain fog, providing practical guidance for symptom management and appropriate medical consultation.
'Brain fog' is a lay term rather than a formal medical diagnosis, used to describe a constellation of cognitive symptoms that affect mental clarity and function. Patients experiencing brain fog typically report:
Difficulty concentrating or maintaining focus on tasks
Memory problems, particularly with short-term recall or word-finding
Mental fatigue or feeling mentally 'sluggish'
Reduced processing speed when thinking or responding
Confusion or difficulty organising thoughts
Lack of mental clarity, often described as thinking through a 'haze'
These symptoms can range from mild and intermittent to more persistent and functionally impairing, affecting work performance, daily activities, and quality of life. Brain fog is not unique to any single condition or medication; it can arise from numerous causes including sleep deprivation, nutritional deficiencies (particularly B vitamins, iron, or vitamin D), dehydration, hormonal changes, chronic stress, depression, anxiety, and various medical conditions such as hypothyroidism or chronic fatigue syndrome.
In the context of weight management treatment, several factors may contribute to cognitive symptoms. Caloric restriction and significant dietary changes can temporarily affect glucose availability to the brain, potentially impacting cognitive function. Rapid weight loss may alter hormone levels and metabolic processes. Additionally, some individuals may experience electrolyte imbalances or dehydration, particularly if experiencing gastrointestinal side effects.
It is important to recognise that brain fog symptoms are subjective and can be influenced by psychological factors, including anxiety about medication side effects or the stress of lifestyle modification. A thorough assessment is necessary to identify the underlying cause and determine appropriate management strategies.
Currently, there is no official, established link between Wegovy (semaglutide) and brain fog in the approved prescribing information or major clinical trial data. The Wegovy SmPC does not list cognitive impairment among its known adverse reactions. The pivotal STEP (Semaglutide Treatment Effect in People with obesity) trials, which formed the basis for Wegovy's regulatory approval, did not identify cognitive impairment or brain fog as significant adverse events. The most commonly reported side effects were gastrointestinal in nature, including nausea, diarrhoea, and vomiting.
However, anecdotal post-marketing reports suggest that some individuals do experience cognitive symptoms whilst taking GLP-1 receptor agonists, though the incidence remains unknown. Several potential mechanisms have been proposed, though robust evidence remains limited:
Indirect metabolic effects: GLP-1 receptors are present in the central nervous system, including areas involved in cognition and mood regulation. Whilst semaglutide's primary therapeutic effects target appetite regulation, the broader neurological impact of sustained GLP-1 receptor activation is not fully characterised.
Hypoglycaemia: Hypoglycaemia is rare (<1%) in patients taking Wegovy as monotherapy. However, patients with type 2 diabetes using Wegovy alongside other glucose-lowering medications (particularly insulin or sulphonylureas) require careful monitoring as the risk may be increased.
Nutritional factors: Reduced food intake and gastrointestinal side effects may lead to inadequate nutrition or hydration, potentially contributing to cognitive symptoms. Deficiencies in essential nutrients can impair brain function.
Dehydration and electrolyte disturbance: Persistent nausea, vomiting, or diarrhoea may result in fluid and electrolyte imbalances, which can manifest as confusion or difficulty concentrating.
It is crucial to note that correlation does not imply causation. Many factors associated with obesity, weight loss, and lifestyle modification may independently contribute to cognitive symptoms, making it challenging to attribute brain fog specifically to Wegovy.
If you experience cognitive symptoms whilst taking Wegovy, several practical strategies may help manage or alleviate brain fog:
Optimise hydration and nutrition: Ensure adequate fluid intake (approximately 6–8 glasses of water daily, about 1.2 litres) as recommended by the NHS Eatwell Guide, and maintain a balanced diet rich in essential nutrients. Focus on:
Complex carbohydrates for sustained glucose supply to the brain
Protein-rich foods to support neurotransmitter production
Omega-3 fatty acids (found in oily fish) for cognitive health
B vitamins, particularly B12 and folate
Iron and vitamin D, if deficient
When rehydrating, be mindful not to exceed recommended sodium intake. Consider consulting a registered dietitian for personalised nutritional guidance during weight loss treatment.
Monitor blood glucose levels: If you have diabetes or experience symptoms suggestive of hypoglycaemia (shakiness, sweating, confusion), regular glucose monitoring is essential. Discuss any concerns with your healthcare provider.
Prioritise sleep hygiene: Aim for 7–9 hours of quality sleep nightly. Establish regular sleep-wake times, create a restful environment, and limit screen exposure before bedtime. Poor sleep significantly impairs cognitive function.
Manage stress: Chronic stress and anxiety can exacerbate cognitive symptoms. Consider stress-reduction techniques such as mindfulness, meditation, gentle exercise (walking, yoga), or cognitive behavioural approaches.
Gradual dose titration: Wegovy is initiated at a low dose (0.25 mg weekly) and gradually increased over 16–20 weeks to the maintenance dose of 2.4 mg. This titration schedule helps minimise side effects. If cognitive symptoms emerge during dose escalation, discuss with your prescriber whether temporarily maintaining the current dose might be appropriate.
Review concurrent medications: Some medications can contribute to cognitive impairment. Your GP or pharmacist can review your complete medication list to identify potential contributors.
Keep a symptom diary: Recording when cognitive symptoms occur, their severity, and associated factors (meals, hydration, sleep, stress) can help identify patterns and triggers, facilitating more targeted management.
Whilst mild, transient cognitive symptoms may resolve with supportive measures, certain situations warrant prompt medical evaluation. Contact your GP or prescribing clinician if you experience:
Severe or worsening cognitive impairment that significantly affects daily functioning, work performance, or safety (e.g., difficulty driving)
Sudden onset of confusion or disorientation
Memory loss that is progressive or concerning
Cognitive symptoms accompanied by other neurological signs such as severe headache, visual disturbances, weakness, numbness, or speech difficulties
Symptoms suggestive of hypoglycaemia (particularly if taking other diabetes medications): shakiness, sweating, rapid heartbeat, confusion, or loss of consciousness
Signs of severe dehydration: reduced urination, dark urine, dizziness, rapid heartbeat, or extreme thirst
Mood changes including significant depression, anxiety, or suicidal thoughts
Persistent symptoms that do not improve with supportive measures after 2–4 weeks
Your healthcare provider will conduct a thorough assessment, which may include:
Clinical history and medication review
Physical examination including neurological assessment
Blood tests to evaluate glucose levels, electrolytes, kidney function, thyroid function, vitamin B12, folate, and other relevant parameters
Blood pressure monitoring
Consideration of alternative diagnoses
Depending on findings, management may involve adjusting the Wegovy dose, temporarily discontinuing treatment, addressing nutritional deficiencies, or investigating other potential causes. Never stop Wegovy abruptly without medical guidance. Your prescriber can provide individualised advice based on your specific circumstances, balancing the benefits of continued weight management treatment against any adverse effects. If cognitive symptoms are severe or persistent despite intervention, alternative weight management strategies may be considered in accordance with NICE guidance.
If you suspect you are experiencing side effects from Wegovy or any medication, you can report these through the MHRA Yellow Card Scheme (yellowcard.mhra.gov.uk), which helps monitor medication safety.
No, brain fog is not listed as an official adverse reaction in Wegovy's Summary of Product Characteristics or pivotal clinical trial data. However, some individuals report cognitive symptoms that may relate to dietary changes, dehydration, or metabolic factors associated with weight loss treatment rather than the medication itself.
Potential causes include caloric restriction affecting glucose availability to the brain, dehydration or electrolyte imbalances from gastrointestinal side effects, nutritional deficiencies (particularly B vitamins, iron, or vitamin D), poor sleep, stress, or rarely hypoglycaemia in patients taking other diabetes medications.
Seek medical advice if you experience severe or worsening cognitive impairment affecting daily functioning, sudden confusion, symptoms suggestive of hypoglycaemia, signs of severe dehydration, accompanying neurological symptoms (severe headache, visual disturbances, weakness), significant mood changes, or persistent symptoms not improving with supportive measures after 2–4 weeks.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.