
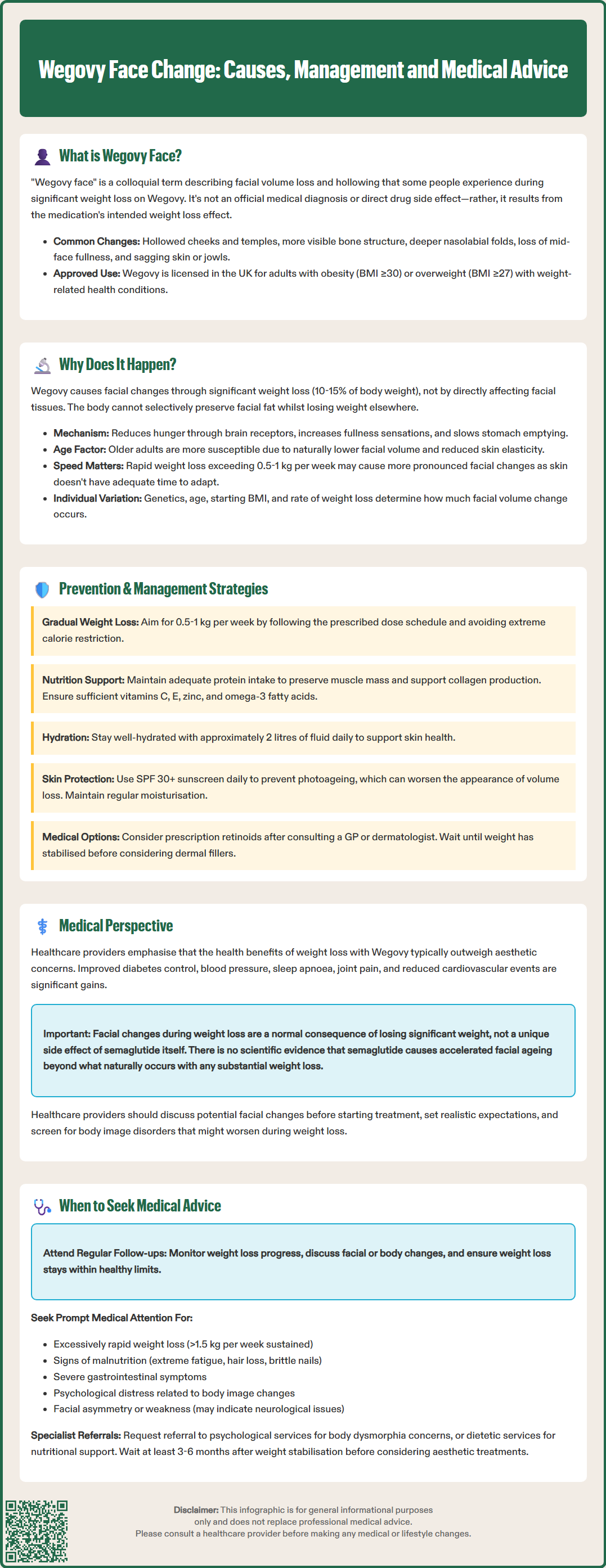
Wegovy face change, colloquially termed 'Ozempic face', describes facial volume loss experienced by some individuals during treatment with Wegovy (semaglutide 2.4 mg) for weight management. This phenomenon—characterised by hollowed cheeks, deepened nasolabial folds, and reduced facial fullness—has gained attention as Wegovy prescriptions increase across the UK. Importantly, these changes are not a direct pharmacological effect of semaglutide but rather reflect the aesthetic consequences of significant weight reduction. Understanding the mechanisms, management strategies, and when to seek medical advice helps patients and clinicians navigate this aspect of obesity treatment whilst prioritising the substantial cardiovascular and metabolic health benefits Wegovy delivers.
Quick Answer: Wegovy face change refers to facial volume loss and hollowing that occurs as a secondary effect of the significant weight reduction facilitated by semaglutide, rather than a direct drug effect on facial tissues.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereWegovy face change, sometimes referred to as 'Ozempic face' or 'semaglutide face', describes the facial volume loss and changes in facial appearance that some individuals experience during treatment with Wegovy (semaglutide 2.4 mg). This phenomenon has gained attention as Wegovy prescriptions have increased following its approval for weight management in the UK.
Wegovy is a glucagon-like peptide-1 (GLP-1) receptor agonist licensed by the MHRA for chronic weight management in adults with obesity (BMI ≥30 kg/m²) or overweight (BMI ≥27 kg/m²) with at least one weight-related comorbidity. The medication works by mimicking the natural hormone GLP-1, which regulates appetite and food intake, leading to significant weight reduction when combined with lifestyle modifications.
Facial changes associated with Wegovy typically manifest as:
Hollowing of the cheeks and temples
Increased prominence of facial bone structure
Deepening of nasolabial folds (lines from nose to mouth)
Loss of facial fullness, particularly in the mid-face region
More pronounced jowls or sagging skin
It is important to note that 'Wegovy face' is not an official medical diagnosis or a listed adverse effect in the Summary of Product Characteristics (SmPC). Lipoatrophy (localised fat loss) is not reported as a specific adverse effect. Rather, 'Wegovy face' represents a colloquial term describing the aesthetic consequences of rapid or substantial weight loss. The facial changes are generally considered a secondary effect of the medication's primary action—significant body weight reduction—rather than a direct pharmacological effect on facial tissues. Understanding this distinction helps patients and healthcare professionals contextualise these changes within the broader framework of weight management therapy.
The facial changes observed with Wegovy treatment are primarily attributable to the substantial weight loss the medication facilitates, rather than a direct effect of semaglutide on facial tissues. Understanding the underlying mechanisms helps clarify why these changes occur and who may be most susceptible.
Mechanism of Weight Loss
Wegovy works through several complementary pathways. As a GLP-1 receptor agonist, semaglutide acts on receptors in the brain's appetite centres, particularly the hypothalamus, reducing hunger and increasing feelings of satiety. It also slows gastric emptying, prolonging the sensation of fullness after meals. Clinical trials have demonstrated that Wegovy can facilitate weight loss of 10-15% of initial body weight over 68 weeks, with some individuals experiencing even greater reductions (Wilding et al., NEJM 2021).
Fat Distribution and Facial Volume
The face contains subcutaneous fat compartments that contribute to facial contours and a youthful appearance. During significant weight loss, the body mobilises fat stores throughout the body, including these facial fat pads. Unfortunately, we cannot selectively preserve facial fat whilst losing weight elsewhere. The rate and extent of facial fat loss varies between individuals based on:
Age: Older adults naturally have less facial volume and reduced skin elasticity
Genetics: Individual fat distribution patterns
Rate of weight loss: Rapid weight reduction (exceeding the NICE-recommended 0.5-1 kg per week) may not allow skin adequate time to adapt
Starting weight: Those with higher initial BMI may experience more dramatic changes, though individual variation is significant
Skin Elasticity Considerations
As we age, collagen and elastin production decreases, reducing the skin's ability to retract after volume loss. Consequently, older patients or those losing weight rapidly may notice more pronounced sagging or hollowing. There is no official link between semaglutide and accelerated skin ageing; the changes reflect the natural consequences of volume depletion in tissues with limited elastic recoil.
Whilst facial volume loss cannot be entirely prevented during significant weight reduction, several strategies may help minimise its appearance and support overall skin health during Wegovy treatment.
Optimising the Rate of Weight Loss
Gradual, steady weight loss allows the skin more time to adapt to changing facial contours. NICE guidance (CG189) recommends a weight loss rate of 0.5-1 kg per week as sustainable and health-promoting. Patients should:
Follow the prescribed Wegovy dose escalation schedule carefully
Work with healthcare professionals to adjust dosing if weight loss is excessively rapid
Maintain realistic expectations about treatment timelines
Combine medication with structured lifestyle modifications rather than extreme calorie restriction
Nutritional Support for Skin Health
Adequate nutrition is essential during weight loss therapy. Key considerations include:
Protein intake: Current evidence suggests maintaining adequate protein intake to preserve lean muscle mass and support collagen synthesis (consult a dietitian for personalised advice aligned with SACN recommendations)
Hydration: Maintain adequate fluid intake (approximately 2 litres daily) for skin turgor
Micronutrients: Ensure sufficient vitamin C (collagen production), vitamin E (antioxidant protection), and zinc (skin repair)
Healthy fats: Include omega-3 fatty acids from oily fish, which support skin barrier function
Skincare and Non-Invasive Approaches
Whilst there is limited evidence that topical treatments can reverse volume loss, maintaining skin health may improve overall appearance:
Sun protection: Daily broad-spectrum SPF 30+ to prevent photoaging
Retinoids: Prescription retinoids require consultation with a GP or dermatologist; over-the-counter retinol products may offer modest benefits
Moisturisation: Regular use of emollients to maintain skin barrier function
Facial exercises: Limited evidence suggests facial muscle training may provide modest improvements, though research quality is low and results are variable
Medical Aesthetic Interventions
For those significantly concerned about facial changes, consultation with a qualified aesthetic practitioner may be appropriate after weight stabilisation. Options include dermal fillers (hyaluronic acid-based products) to restore volume, though these should only be considered once weight has plateaued to ensure optimal, lasting results.
Healthcare professionals recognise that facial changes during weight loss treatment represent a complex interplay between the substantial health benefits of obesity treatment and the aesthetic concerns that may affect treatment adherence and psychological wellbeing.
Clinical Significance and Health Priorities
From a medical standpoint, the cardiovascular, metabolic, and mortality benefits of significant weight reduction typically far outweigh aesthetic considerations. Preliminary results from the SELECT trial suggest that semaglutide may reduce major adverse cardiovascular events in people with established cardiovascular disease and obesity. Weight loss achieved through Wegovy treatment has been shown to improve:
Type 2 diabetes control and potentially induce remission
Blood pressure and lipid profiles
Obstructive sleep apnoea severity
Joint pain and mobility
Quality of life measures across multiple domains
NICE guidance (TA875) recommends Wegovy as a cost-effective intervention for eligible patients, emphasising these broader health outcomes. Healthcare professionals must balance discussing potential aesthetic changes with reinforcing the significant health improvements that treatment facilitates.
Psychological and Quality of Life Considerations
Research indicates that body image concerns can significantly impact treatment adherence. Clinical experience suggests that a proportion of patients express concerns about facial appearance changes, though the exact prevalence is not well-established in the literature. Healthcare professionals should:
Proactively discuss potential facial changes during treatment initiation
Normalise these changes as a common consequence of significant weight loss
Screen for body dysmorphia or disordered eating patterns that may be exacerbated
Provide realistic expectations about the timeline and extent of changes
Evidence Base and Research Gaps
Currently, there is no official link established between semaglutide and accelerated facial ageing beyond that expected from weight loss itself. Prospective studies specifically examining facial changes, their predictors, and patient-reported outcomes are limited. The phenomenon has been primarily documented through anecdotal reports and social media discussion rather than rigorous clinical investigation. Further research is needed to quantify the prevalence, identify risk factors, and develop evidence-based mitigation strategies.
Whilst facial volume changes during Wegovy treatment are generally benign and expected with significant weight loss, certain situations warrant medical review to ensure patient safety and treatment optimisation.
Routine Monitoring and Follow-Up
Patients prescribed Wegovy should have regular follow-up appointments according to local weight management service protocols, which typically include:
Regular reviews during dose escalation
Ongoing monitoring once on maintenance dose
Assessment of weight loss progress, tolerability, and comorbidity improvement
During these appointments, patients should feel comfortable discussing any concerns about facial or body changes. Healthcare professionals can provide reassurance, adjust treatment plans if necessary, and ensure that weight loss remains within healthy parameters.
Signs Requiring Prompt Medical Attention
Patients should contact their GP or prescribing clinician if they experience:
Excessively rapid weight loss (>1.5 kg per week sustained over several weeks)
Signs of malnutrition: extreme fatigue, hair loss, brittle nails, persistent dizziness
Severe gastrointestinal symptoms preventing adequate nutrition or hydration
Psychological distress related to body image changes, including anxiety, depression, or disordered eating behaviours
Facial asymmetry or weakness, which could indicate neurological issues unrelated to weight loss and requires urgent assessment
Patients should report any suspected adverse reactions to Wegovy via the MHRA Yellow Card Scheme (yellowcard.mhra.gov.uk), even if the effect is not listed in the product information.
Psychological Support and Referral
Significant body composition changes can trigger or exacerbate body dysmorphic disorder or eating disorders. Healthcare professionals should consider referral to:
Psychological services if patients exhibit signs of body dysmorphia or significant distress
Dietetic services for optimised nutritional support during weight loss
Specialist weight management services for complex cases
Aesthetic Medicine Consultation
For patients whose facial changes significantly impact quality of life after weight stabilisation, referral to a qualified aesthetic practitioner may be appropriate. Patients should be advised to:
Wait until weight has stabilised for at least 3-6 months
Seek practitioners registered with appropriate professional bodies (e.g., General Medical Council for doctors)
Understand that aesthetic interventions are typically not available through the NHS and involve private costs
Ultimately, open communication between patients and healthcare providers ensures that both the medical benefits of weight loss and the psychological wellbeing of patients are appropriately addressed throughout Wegovy treatment.
No, Wegovy face change is not a direct pharmacological effect of semaglutide but rather a secondary consequence of the significant weight loss the medication facilitates. Facial volume loss occurs as subcutaneous fat is mobilised throughout the body, including facial fat compartments.
Facial volume loss cannot be entirely prevented during significant weight reduction, but gradual weight loss (0.5–1 kg per week), adequate protein intake, and maintaining skin health through hydration and sun protection may help minimise its appearance.
Contact your GP if you experience excessively rapid weight loss (>1.5 kg per week sustained), signs of malnutrition, severe gastrointestinal symptoms, or significant psychological distress related to body image changes. Facial asymmetry or weakness requires urgent assessment.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.