
What is the once a week injection for weight loss? These injections are GLP-1 receptor agonists, a class of medications administered subcutaneously once weekly to support weight management. The most commonly prescribed option is semaglutide (Wegovy), with tirzepatide representing another once-weekly alternative. Originally developed for type 2 diabetes treatment, these medications demonstrated substantial weight loss effects, leading to FDA approval specifically for chronic weight management in adults with obesity or those who are overweight with weight-related health conditions. The self-administered injection uses a pre-filled pen device and must be combined with a reduced-calorie diet and increased physical activity as part of a comprehensive weight management program.
Quick Answer: The once-a-week injection for weight loss is a GLP-1 receptor agonist medication, most commonly semaglutide (Wegovy), administered subcutaneously to support weight management alongside diet and exercise.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereThe once-a-week injection for weight loss refers to a class of medications called GLP-1 receptor agonists (glucagon-like peptide-1 agonists), which are administered subcutaneously once weekly to support weight management. The most commonly prescribed medication in this category is semaglutide (marketed as Wegovy specifically for weight management). Another once-weekly option is tirzepatide, a dual GIP/GLP-1 receptor agonist that has shown significant weight loss effects. For comparison, liraglutide (Saxenda) is a related medication but requires daily rather than weekly injections.
These medications were originally developed to treat type 2 diabetes (where semaglutide is marketed as Ozempic), but researchers observed substantial weight loss as a secondary effect. This led to their approval specifically for chronic weight management in adults with obesity or those who are overweight with weight-related health conditions. In the UK, the Medicines and Healthcare products Regulatory Agency (MHRA) has approved semaglutide for weight management, and the National Institute for Health and Care Excellence (NICE) has issued guidance on its use within the NHS.
The injection is self-administered using a pre-filled pen device, similar to insulin pens used by people with diabetes. Patients typically inject the medication into the abdomen, thigh, or upper arm once weekly on the same day each week. The treatment is designed to be used alongside a reduced-calorie diet and increased physical activity as part of a comprehensive weight management programme, rather than as a standalone solution.
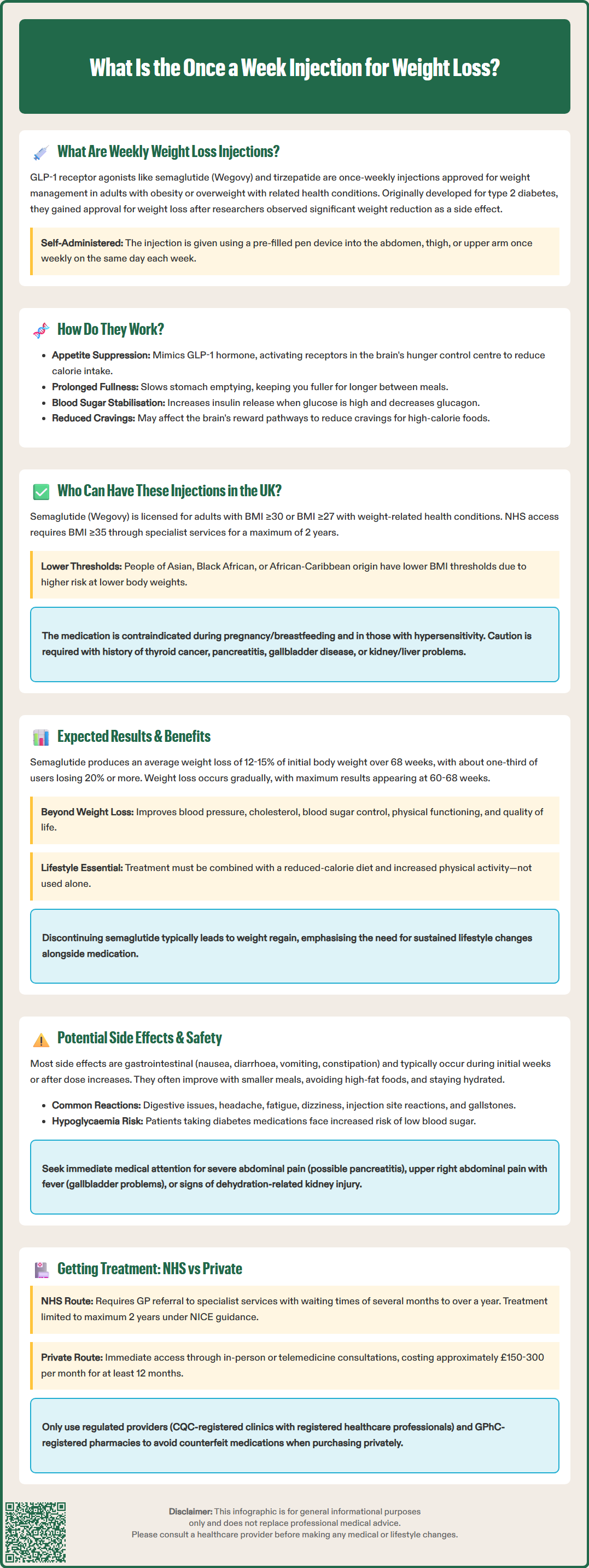
Weekly weight loss injections work by mimicking the action of glucagon-like peptide-1 (GLP-1), a naturally occurring hormone produced in the intestines that plays a crucial role in appetite regulation and glucose metabolism. When you eat, GLP-1 is released and sends signals to various parts of the body to help control blood sugar and feelings of fullness.
The mechanism of action involves several pathways:
Appetite suppression: GLP-1 receptor agonists act on receptors in the brain's hypothalamus, the region responsible for hunger and satiety signals. By activating these receptors, the medication reduces appetite and increases feelings of fullness after eating, leading to decreased caloric intake.
Delayed gastric emptying: These medications slow down the rate at which food leaves the stomach and enters the small intestine. This prolonged gastric emptying contributes to sustained feelings of fullness and reduces the frequency of hunger between meals. This effect may also impact the absorption of certain oral medications, so patients should discuss all current medications with their healthcare provider.
Blood glucose regulation: Although primarily used for weight loss in non-diabetic individuals, GLP-1 agonists also enhance insulin secretion when blood glucose levels are elevated and suppress glucagon release, helping to stabilize blood sugar levels.
Reduced food cravings: Emerging evidence suggests these medications may reduce cravings for high-calorie, palatable foods by affecting reward pathways in the brain, though more research is needed to fully understand this mechanism.
The once-weekly formulation of semaglutide has been specifically engineered to have a longer half-life than natural GLP-1, which is rapidly broken down in the body within minutes. This extended duration of action allows for convenient weekly dosing while maintaining therapeutic drug levels throughout the week.
In the UK, eligibility for once-weekly weight loss injections is determined by both licensing criteria (from the MHRA) and NHS access criteria (from NICE), which are more restrictive.
MHRA licensing criteria for semaglutide (Wegovy) include adults with:
A body mass index (BMI) of 30 kg/m² or above (obesity), or
A BMI of 27 kg/m² or above with at least one weight-related comorbidity (such as hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease)
NHS access via NICE guidance (TA875) is more restrictive and recommends semaglutide only:
Within specialist weight management services
For adults with a BMI of 35 kg/m² or above (or lower for certain ethnic groups)
With at least one weight-related comorbidity
For a maximum treatment duration of 2 years
For individuals of Asian, Black African, or African-Caribbean family origin, lower BMI thresholds may apply due to increased risk of weight-related complications at lower body weights.
Precautions and special warnings include:
Pregnancy or breastfeeding (contraindicated)
Hypersensitivity to the active substance or excipients (contraindicated)
Personal or family history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2 (special warning)
History of pancreatitis (caution)
Gallbladder disease (caution)
Diabetic retinopathy in patients with diabetes (caution)
Moderate to severe renal or hepatic impairment (caution)
Some GLP-1 medications are now approved for adolescents with obesity in the UK, though NHS access may be limited. Wegovy is approved for adolescents aged 12 years and older with body weight above 60 kg and obesity (BMI corresponding to ≥30 kg/m² in adults).
Before prescribing, healthcare professionals should conduct a comprehensive assessment including medical history, current medications, and discussion of realistic weight loss goals. Patients must also demonstrate commitment to lifestyle modifications, as the medication is intended to complement—not replace—dietary changes and increased physical activity.
Clinical trials have demonstrated substantial weight loss outcomes with once-weekly semaglutide injections, making them among the most effective pharmacological treatments currently available for obesity management. Understanding realistic expectations is essential for patients considering this treatment option.
Weight loss outcomes from clinical trials:
The landmark STEP (Semaglutide Treatment Effect in People with obesity) trials showed that participants using semaglutide 2.4 mg weekly achieved an average weight loss of 12-15% of their initial body weight over 68 weeks, compared to 2-3% in placebo groups. Some individuals experienced even greater weight loss, with approximately one-third losing 20% or more of their starting weight. These results represent significantly greater weight loss than achieved with older weight loss medications.
Additional health benefits beyond weight loss include:
Improved cardiovascular risk factors: Reductions in blood pressure, cholesterol levels, and inflammatory markers
Better glycemic control: Particularly beneficial for individuals with prediabetes or type 2 diabetes
Enhanced quality of life: Improvements in physical functioning, mobility, and psychological well-being
Reduced risk of weight-related complications: Potential decreased risk of developing type 2 diabetes, cardiovascular disease, and other obesity-related conditions
It is important to note that weight loss typically occurs gradually, with most significant results appearing after several months of treatment. The medication is started at a low dose and gradually increased over 16-20 weeks to minimize side effects and allow the body to adjust. Maximum weight loss is generally achieved between 60-68 weeks of treatment.
Weight maintenance considerations: Evidence suggests that discontinuing the medication often leads to weight regain, highlighting the importance of sustained lifestyle modifications. Within the NHS, NICE recommends treatment for up to 2 years, after which continuation would depend on local policies and individual clinical assessment.
While once-weekly weight loss injections are generally well-tolerated, patients should be aware of potential side effects and safety considerations before starting treatment. Most adverse effects are gastrointestinal in nature and tend to be most pronounced during the initial weeks of treatment or following dose increases.
Common side effects (affecting more than 1 in 10 people):
Nausea: The most frequently reported side effect, usually mild to moderate and improving over time
Diarrhea: May occur intermittently, particularly after dose escalation
Vomiting: Less common than nausea but can occur, especially if large meals are consumed
Constipation: Some patients experience reduced bowel movements
Abdominal pain or discomfort: Generally mild and transient
Headache: Usually resolves without intervention
Dyspepsia, abdominal distension, and flatulence: Common digestive symptoms
Fatigue and dizziness: May affect daily activities
Injection site reactions: Including redness, itching, or bruising
Cholelithiasis (gallstones): More common with significant weight loss
These gastrointestinal symptoms can often be managed by eating smaller, more frequent meals, avoiding high-fat foods, and staying well-hydrated. If symptoms are severe or persistent, patients should contact their healthcare provider, as dose adjustment may be necessary.
Serious but rare side effects requiring immediate medical attention:
Pancreatitis: Severe, persistent abdominal pain radiating to the back, often accompanied by vomiting. Stop the medication and seek urgent medical care.
Gallbladder problems: Symptoms include upper right abdominal pain, fever, and jaundice.
Hypoglycemia: Particularly in patients taking other diabetes medications; symptoms include shakiness, sweating, confusion, and rapid heartbeat.
Acute kidney injury: Usually related to severe dehydration from vomiting or diarrhea; maintain adequate fluid intake.
Allergic reactions: Rash, itching, difficulty breathing, or swelling of face, lips, or throat.
Diabetic retinopathy complications: Patients with diabetes should report any visual changes promptly and maintain regular eye examinations.
Important safety considerations: Animal studies showed an increased risk of thyroid C-cell tumors with GLP-1 receptor agonists, though no causal relationship has been established in humans. As a precaution, patients should report any neck lumps, hoarseness, or difficulty swallowing to their healthcare provider promptly.
Patients are encouraged to report any suspected side effects to the MHRA Yellow Card Scheme, which helps monitor the safety of medications.
Access to once-weekly weight loss injections in the UK is available through both NHS and private healthcare routes, though availability, eligibility criteria, and costs differ significantly between these pathways.
NHS access:
NHS provision of weight loss injections is currently limited due to high demand and supply constraints. NICE has recommended semaglutide for weight management in specialist weight management services, but implementation varies across different regions and Integrated Care Boards (ICBs). Under NICE guidance (TA875), treatment is typically limited to a maximum of 2 years. Patients typically need:
Referral from their GP to a specialist weight management service
Assessment by a multidisciplinary team including dietitians, physicians, and sometimes psychologists
Participation in a structured weight management program including dietary counseling and physical activity support
Regular monitoring and follow-up appointments
Waiting times for NHS weight management services can be substantial, ranging from several months to over a year in some areas. The NHS prioritizes patients with the highest clinical need, including those with significant weight-related comorbidities.
Private access:
Private clinics and online prescribing services offer more immediate access to weight loss injections, though at considerable cost. Private treatment typically involves:
Initial consultation with a prescribing clinician (in-person or via telemedicine)
Medical assessment to confirm eligibility and safety
Prescription and supply of medication
Ongoing monitoring and support (varies by provider)
Costs: Private treatment with semaglutide typically costs approximately $150-300 per month, depending on the dose and provider. This represents a significant ongoing expense, as treatment is usually required for at least 12 months and potentially longer for weight maintenance.
Important considerations: Patients should ensure any private provider is regulated by the Care Quality Commission (CQC) and that prescribers are registered healthcare professionals. Medications should only be obtained from General Pharmaceutical Council (GPhC) registered pharmacies to avoid counterfeit or unauthorized products, particularly when purchasing online. All patients, whether accessing treatment through NHS or privately, should receive comprehensive support including dietary advice and lifestyle counseling, as medication alone is insufficient for sustainable weight management. If you experience concerning symptoms or have questions about your treatment, contact your prescribing clinician or GP promptly.
Clinical trials showed participants using semaglutide 2.4 mg weekly achieved an average weight loss of 12-15% of their initial body weight over 68 weeks, with approximately one-third losing 20% or more. Individual results vary and depend on adherence to diet and exercise modifications.
Once-weekly GLP-1 receptor agonists are FDA-approved and generally well-tolerated, though common side effects include nausea, diarrhea, and constipation. Rare serious risks include pancreatitis, gallbladder problems, and contraindication in those with personal or family history of medullary thyroid carcinoma, requiring ongoing medical supervision.
Yes, medications like semaglutide (Wegovy) and tirzepatide require a prescription from a licensed healthcare provider. Patients must meet specific BMI and health criteria, undergo medical assessment, and receive ongoing monitoring throughout treatment.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.