
Tirzepatide, marketed as Mounjaro, is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist licensed in the UK for type 2 diabetes mellitus and chronic weight management. Beyond its primary metabolic effects, emerging evidence suggests tirzepatide may reduce systemic inflammation, a key contributor to cardiovascular disease and metabolic complications. Whilst not licensed as an anti-inflammatory medicine, reductions in inflammatory markers such as high-sensitivity C-reactive protein (hs-CRP) and interleukin-6 (IL-6) have been observed in clinical trials, largely correlating with substantial weight loss and improved glycaemic control. This article examines the mechanisms, clinical evidence, and implications of tirzepatide's potential anti-inflammatory properties.
Quick Answer: Tirzepatide appears to reduce systemic inflammation markers, though these effects are largely secondary to substantial weight loss and improved glycaemic control rather than direct anti-inflammatory action.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereTirzepatide is a novel glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist licensed in the UK for the treatment of type 2 diabetes mellitus and, more recently, for chronic weight management in adults with obesity or overweight with weight-related comorbidities. Marketed under the brand name Mounjaro, tirzepatide represents the first dual incretin receptor agonist approved by the Medicines and Healthcare products Regulatory Agency (MHRA).
The drug works through a unique dual mechanism of action. By activating both GIP and GLP-1 receptors, tirzepatide enhances glucose-dependent insulin secretion from pancreatic beta cells, suppresses inappropriate glucagon release, slows gastric emptying, and reduces appetite through central nervous system pathways. This combined action results in improved glycaemic control and substantial weight reduction, with clinical trials demonstrating HbA1c reductions of up to 2.5% and weight loss exceeding 20% in some patients.
Tirzepatide is administered once weekly via subcutaneous injection, with doses ranging from 2.5 mg to 15 mg depending on therapeutic goals and tolerability. The medication is initiated at a low dose and gradually titrated upwards over several weeks to minimise gastrointestinal adverse effects. NICE guidance (NG28) supports the use of incretin-based therapies in type 2 diabetes management when metformin and other oral agents prove insufficient. For weight management, NICE restricts NHS use to specialist weight management services for patients meeting specific BMI thresholds.
It is important to note that tirzepatide is not indicated for the treatment of type 1 diabetes or diabetic ketoacidosis. Beyond its primary metabolic effects, emerging research suggests tirzepatide may have effects on systemic inflammation, a contributor to cardiovascular disease and metabolic complications in patients with diabetes and obesity, though this remains an exploratory area of investigation.
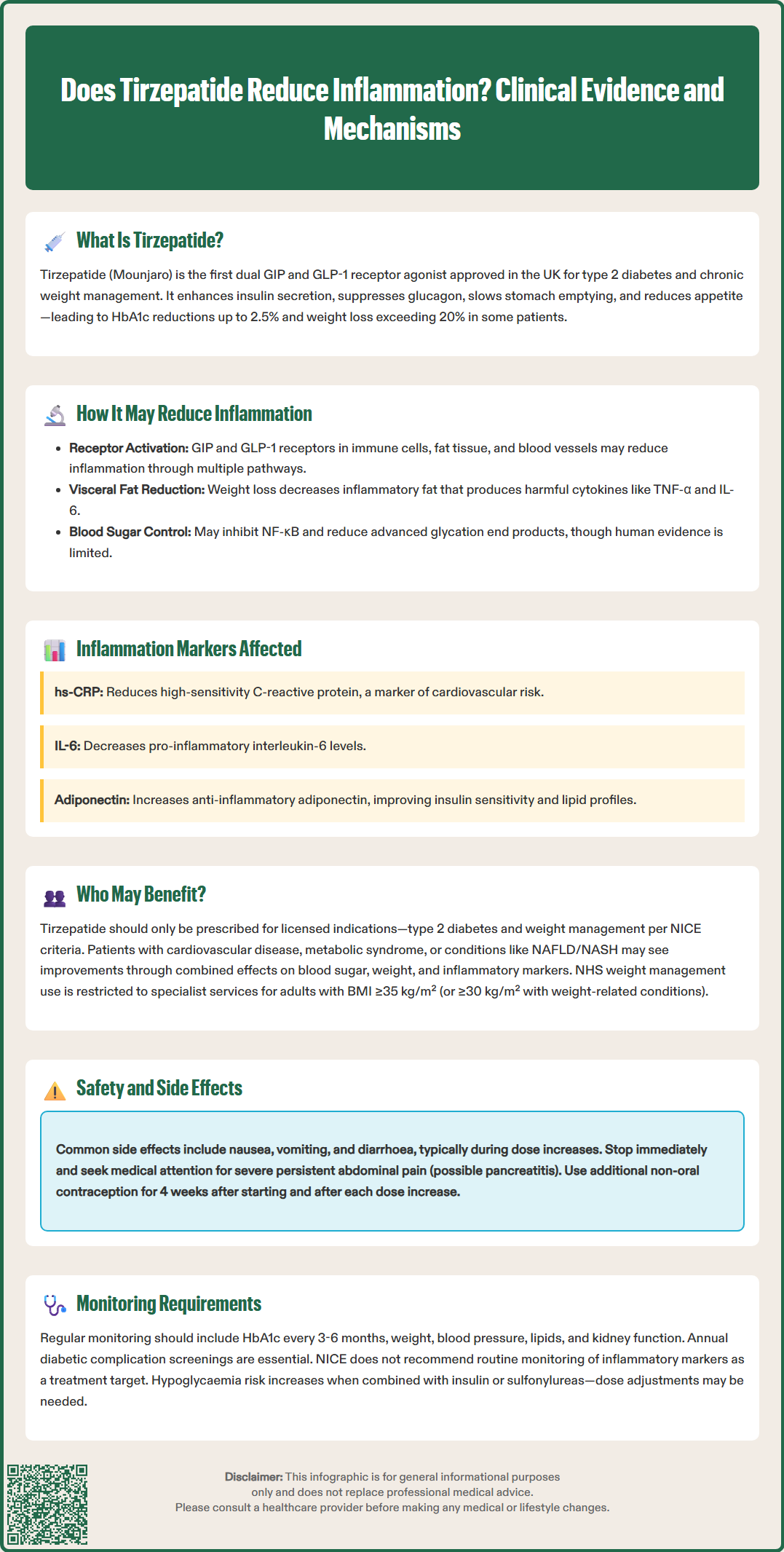
The potential anti-inflammatory properties of tirzepatide appear to stem from multiple interconnected mechanisms. Both GIP and GLP-1 receptors are expressed not only in pancreatic tissue but also in immune cells, adipose tissue, and vascular endothelium, though much of this evidence comes from preclinical studies. Activation of these receptors may modulate inflammatory pathways through several routes, including reduction of adipose tissue inflammation, improvement in endothelial function, and direct effects on immune cell activity.
Weight loss itself represents a significant anti-inflammatory intervention. Excess adipose tissue, particularly visceral fat, functions as an active endocrine organ secreting pro-inflammatory cytokines such as tumour necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1). The substantial weight reduction achieved with tirzepatide therapy leads to decreased adipose tissue mass and consequently reduced production of these inflammatory mediators. Clinical studies, including the SURMOUNT-1 trial, have shown reductions in markers of systemic inflammation alongside weight loss, though it remains unclear whether these effects are independent of weight reduction.
Preclinical research suggests GLP-1 receptor activation may inhibit nuclear factor kappa B (NF-κB), a regulator of inflammatory gene expression, though human evidence for this specific mechanism is limited. Additionally, improved glycaemic control reduces the formation of advanced glycation end products (AGEs), which trigger inflammatory responses through receptor-mediated pathways. The GIP component of tirzepatide may contribute additional effects through modulation of adipocyte function and lipid metabolism.
While the SURPASS clinical trial programme has provided valuable data on metabolic outcomes, studies specifically examining inflammatory parameters are more limited. It is important to emphasise that tirzepatide is not licensed as an anti-inflammatory medicine, and any such effects should be considered secondary to its primary metabolic actions.
Several inflammatory biomarkers have been evaluated in patients receiving tirzepatide therapy, providing insight into the drug's potential effects on inflammation. High-sensitivity C-reactive protein (hs-CRP), an acute-phase reactant produced by the liver in response to inflammatory cytokines, serves as a widely used marker of systemic inflammation and cardiovascular risk. Some studies have demonstrated reductions in hs-CRP levels among patients treated with tirzepatide, though the magnitude varies between studies and patient populations.
In the SURMOUNT-1 trial, for example, tirzepatide treatment was associated with reductions in hs-CRP compared to placebo, correlating with the degree of weight loss achieved. Research has also examined changes in specific cytokines and adipokines. Interleukin-6 (IL-6), a pro-inflammatory cytokine elevated in obesity and type 2 diabetes, may decrease during tirzepatide treatment. Conversely, adiponectin—an anti-inflammatory adipokine with insulin-sensitising properties that is characteristically reduced in obesity—often increases with therapy. These changes in the inflammatory profile generally correlate with improvements in metabolic parameters including insulin sensitivity, lipid profiles, and blood pressure.
Tumour necrosis factor-alpha (TNF-α) and other markers of immune activation have shown variable responses in clinical studies. It is important to note that whilst these biomarker improvements are observed, there is no definitive evidence establishing tirzepatide as a primary anti-inflammatory agent independent of its metabolic effects. Furthermore, routine monitoring of inflammatory markers is not recommended by NICE as a target of therapy.
The clinical significance of inflammatory marker reductions extends beyond laboratory values. Chronic low-grade inflammation contributes to cardiovascular disease, non-alcoholic fatty liver disease (NAFLD), and other complications of obesity and diabetes. The SURPASS-CVOT study is currently evaluating major adverse cardiovascular events, though results have not yet been reported. Further research is needed to fully characterise the contribution of any anti-inflammatory effects to clinical outcomes.
Whilst tirzepatide is primarily prescribed for glycaemic control in type 2 diabetes and weight management, certain patient populations may derive particular benefit from its metabolic effects, which may include reductions in inflammatory markers. It is essential to emphasise that tirzepatide should only be prescribed according to its licensed indications and NICE-approved criteria, not for off-label anti-inflammatory purposes.
Individuals with type 2 diabetes and established cardiovascular disease represent a key group, as chronic inflammation plays a role in atherosclerotic progression. The combination of improved glycaemic control, weight reduction, and associated changes in inflammatory markers may contribute to overall metabolic improvement in this high-risk population.
Patients with metabolic syndrome—characterised by central obesity, insulin resistance, dyslipidaemia, and hypertension—often exhibit elevated inflammatory markers. Tirzepatide's effects on weight, glucose metabolism, lipids, and blood pressure may help address the complex pathophysiology of this condition, though prescribing should follow NICE guidance for either type 2 diabetes (NG28) or weight management.
For weight management, NICE restricts NHS use to specialist weight management services for adults with a BMI of at least 35 kg/m² (or ≥30 kg/m² with weight-related comorbidities) and specific eligibility criteria, including stopping rules if insufficient weight loss is achieved.
Individuals with non-alcoholic fatty liver disease (NAFLD) or non-alcoholic steatohepatitis (NASH) may experience improvements as weight loss can reduce hepatic steatosis and inflammation, though tirzepatide is not licensed specifically for liver disease treatment in the UK. Similarly, patients with obesity-related complications including obstructive sleep apnoea, polycystic ovary syndrome (PCOS), and osteoarthritis may experience symptomatic improvement alongside weight reduction, but these are not primary indications.
Healthcare professionals should conduct comprehensive cardiovascular and metabolic risk assessment when considering tirzepatide therapy, ensuring patients meet eligibility criteria for NHS prescribing based on either diabetes management or specialist weight management service criteria.
Tirzepatide is generally well-tolerated, but patients and healthcare professionals must be aware of potential adverse effects and appropriate monitoring requirements. The most common side effects are gastrointestinal, including nausea, vomiting, diarrhoea, constipation, and abdominal discomfort. These symptoms typically occur during dose escalation and often diminish over time. Patients should be advised to eat smaller meals, avoid high-fat foods, and stay well-hydrated. If gastrointestinal symptoms are severe or persistent, dose reduction or temporary treatment interruption may be necessary.
Hypoglycaemia risk is generally low with tirzepatide monotherapy due to its glucose-dependent mechanism of action. However, when combined with insulin or sulfonylureas, the risk increases substantially. Dose reduction of concomitant glucose-lowering medications may be required, and patients should be educated about hypoglycaemia recognition and management. Blood glucose monitoring frequency should be individualised based on overall diabetes regimen and stability.
Patients should be informed that tirzepatide may reduce the effectiveness of oral contraceptives, particularly during treatment initiation and dose increases. Additional non-oral contraceptive methods are recommended for 4 weeks after starting treatment and after each dose increase. Tirzepatide is not recommended during pregnancy or breastfeeding and should be discontinued if pregnancy occurs or is planned.
Acute pancreatitis has been reported with GLP-1 receptor agonists. If pancreatitis is suspected, tirzepatide should be discontinued; if confirmed, treatment should not be restarted. Patients should be counselled to seek immediate medical attention if they experience severe, persistent abdominal pain radiating to the back. Caution is advised in patients with severe gastrointestinal disease or gastroparesis.
Rapid improvement in glycaemic control has been associated with temporary worsening of diabetic retinopathy. Appropriate retinopathy screening should be ensured, particularly in patients with a history of retinopathy. Gallbladder disease, including cholelithiasis and cholecystitis, occurs more frequently with significant weight loss and has been reported in clinical trials.
Monitoring during treatment should include regular assessment of glycaemic control (HbA1c every 3–6 months), weight, blood pressure, and lipid profile. Renal function should be monitored, particularly in patients experiencing significant gastrointestinal symptoms with potential dehydration. Annual diabetic complications screening, including retinopathy assessment and foot examination, should continue as per NICE guidelines. Healthcare professionals should provide ongoing support for lifestyle modification, as tirzepatide is most effective when combined with dietary changes and increased physical activity.
Patients should be advised to report suspected adverse reactions to the MHRA through the Yellow Card Scheme.
No, tirzepatide is not licensed as an anti-inflammatory medicine in the UK. It is approved by the MHRA for type 2 diabetes mellitus and chronic weight management, with any anti-inflammatory effects considered secondary to its primary metabolic actions.
Clinical studies have shown reductions in high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), and other inflammatory markers during tirzepatide therapy, largely correlating with weight loss and improved glycaemic control.
No, NICE does not recommend routine monitoring of inflammatory markers as a target of tirzepatide therapy. Standard monitoring should focus on HbA1c, weight, blood pressure, lipid profile, and renal function.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.