
Glucagon-like peptide-1 (GLP-1) is an incretin hormone that plays a crucial role beyond blood sugar control, exerting significant effects within the brain. GLP-1 receptors are distributed throughout the central nervous system, influencing appetite regulation, reward processing, learning, and memory. When GLP-1 binds to these brain receptors, it triggers signalling cascades that affect neuronal activity and cellular survival. Understanding how GLP-1 works in the brain has become increasingly relevant as GLP-1 receptor agonist medications—licensed in the UK for type 2 diabetes and weight management—produce neurological effects including appetite suppression and potential neuroprotective benefits. This article explores the mechanisms through which GLP-1 influences brain function and the clinical implications for patients.
Quick Answer: GLP-1 works in the brain by binding to GLP-1 receptors distributed across multiple regions, triggering signalling cascades that suppress appetite, influence reward processing, and may protect neurons from damage.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereGlucagon-like peptide-1 (GLP-1) is an incretin hormone primarily produced by enteroendocrine L-cells in the distal small intestine and colon. It is also produced in the brain by preproglucagon (PPG) neurons in the nucleus tractus solitarius (NTS), which project to the hypothalamus and other brain regions. Whilst its classical role involves stimulating insulin secretion in response to food intake, emerging research has revealed that GLP-1 exerts significant effects beyond glucose regulation. The hormone acts through GLP-1 receptors (GLP-1R), which are G-protein coupled receptors distributed throughout the body, including various regions of the central nervous system.
The presence of GLP-1 receptors in the brain has prompted considerable scientific interest in understanding how this hormone influences neurological function. These receptors are found in areas critical for appetite regulation, reward processing, learning, and memory. When GLP-1 binds to its receptors in neural tissue, it triggers intracellular signalling cascades that influence neuronal activity, synaptic plasticity, and cellular survival mechanisms.
Why brain-based GLP-1 activity matters clinically:
It helps explain the appetite-suppressing effects of GLP-1 receptor agonist medications
It may contribute to neuroprotective mechanisms being investigated in neurodegenerative diseases
It potentially influences cognitive processes and mood regulation, though human evidence remains limited
It provides insight into the gut-brain axis and metabolic-neurological interactions
Understanding GLP-1's cerebral actions has become particularly relevant as GLP-1 receptor agonists—originally developed for type 2 diabetes—are now also licensed for weight management in the UK (NICE TA875, TA664). The neurological effects of these medications extend beyond simple appetite suppression, potentially offering therapeutic benefits for conditions affecting brain health. However, patients should be aware that whilst preclinical research is promising, many neuroprotective applications remain investigational rather than established clinical practice, with no current MHRA approval for neurological indications.
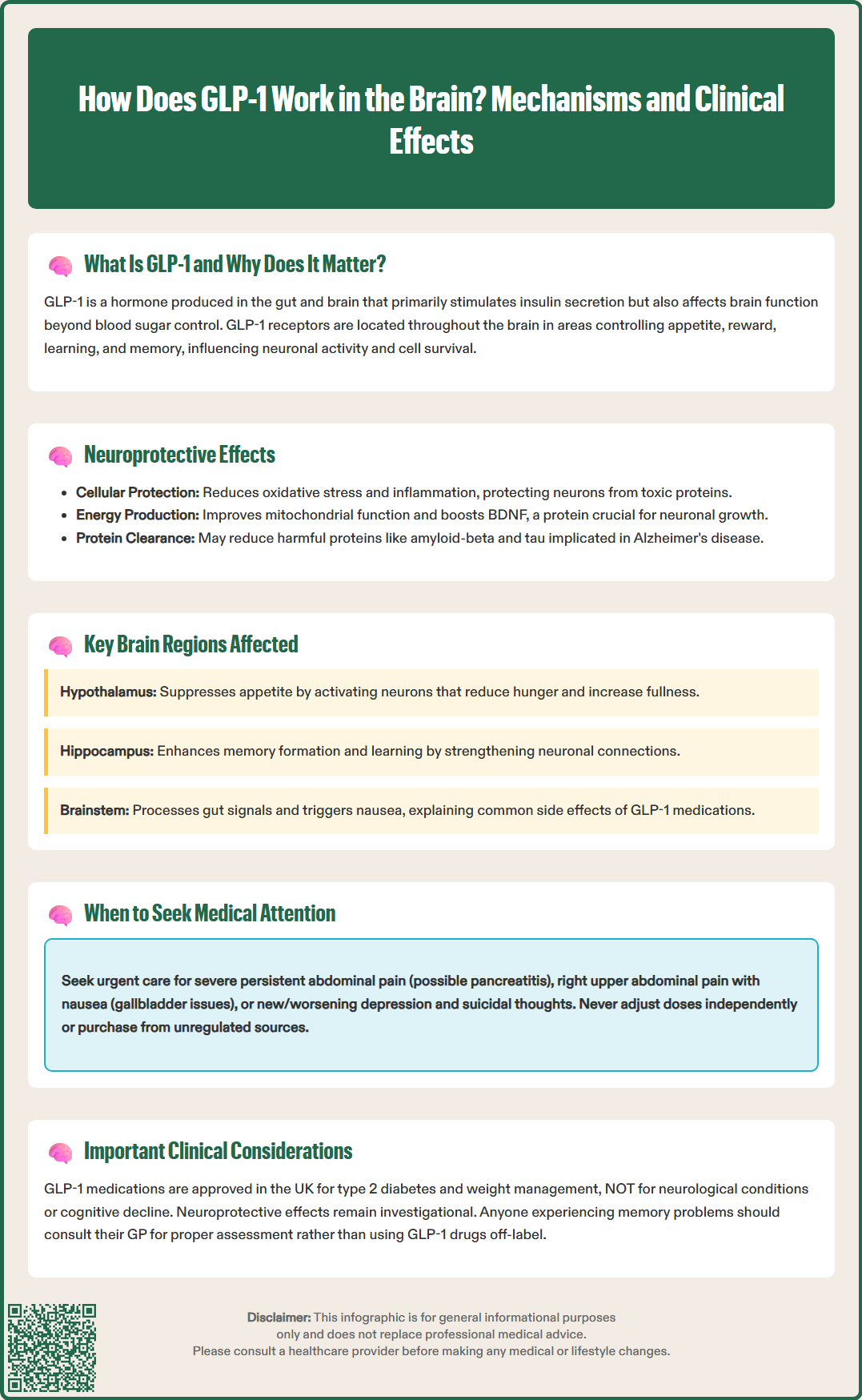
Preclinical research has demonstrated that GLP-1 receptor activation may confer neuroprotective effects through multiple mechanisms. These include reducing oxidative stress, decreasing neuroinflammation, promoting neuronal survival, and enhancing synaptic plasticity. Laboratory studies suggest that GLP-1 receptor agonists can protect neurons from damage caused by toxic protein aggregates, excitotoxicity, and metabolic stress—processes implicated in neurodegenerative conditions such as Alzheimer's disease and Parkinson's disease.
The neuroprotective mechanisms appear to involve several pathways. In experimental models, GLP-1 receptor activation stimulates cyclic AMP (cAMP) production and activates protein kinase A (PKA), which subsequently influences gene transcription and cellular metabolism. This signalling cascade can enhance mitochondrial function, improve cellular energy production, and activate anti-apoptotic (cell survival) pathways. Additionally, preclinical evidence suggests GLP-1 may promote the production of brain-derived neurotrophic factor (BDNF), a protein essential for neuronal growth, differentiation, and long-term memory formation.
Potential cognitive benefits under investigation:
Improved learning and memory consolidation
Enhanced neuroplasticity and synaptic function
Reduced accumulation of pathological proteins (amyloid-beta, tau)
Protection against vascular damage affecting brain tissue
Clinical trials exploring GLP-1 receptor agonists for Alzheimer's disease and other neurodegenerative conditions are ongoing, but evidence in humans remains inconclusive and mixed. It is crucial to emphasise that there is no official link establishing GLP-1 medications as approved treatments for dementia or cognitive decline. The MHRA has not licensed these drugs for neuroprotective indications, and patients should not discontinue established treatments or use GLP-1 agonists off-label for cognitive concerns without specialist neurological assessment. Anyone experiencing memory problems or cognitive changes should consult their GP for appropriate investigation, which may include cognitive screening, blood tests, and neuroimaging as per NICE guidance (NG97). Referral to memory services may be indicated following initial assessment.
GLP-1 receptors are distributed across multiple brain regions, each contributing to distinct physiological and behavioural functions. The hypothalamus represents a primary site of GLP-1 action, particularly in areas regulating energy homeostasis and appetite. Within the hypothalamus, GLP-1 receptors are concentrated in the arcuate nucleus and paraventricular nucleus—regions that integrate peripheral metabolic signals and coordinate feeding behaviour. When GLP-1 binds to receptors in these areas, it activates anorexigenic (appetite-suppressing) neurons whilst inhibiting orexigenic (appetite-stimulating) pathways, contributing to reduced food intake and increased satiety.
Beyond the hypothalamus, GLP-1 receptors are found in the hippocampus, a structure critical for memory formation and spatial learning. Activation of hippocampal GLP-1 receptors has been associated with enhanced long-term potentiation (LTP)—a cellular mechanism underlying learning and memory. This may explain observations that GLP-1 receptor agonists can improve performance on memory tasks in experimental models.
The brainstem, particularly the area postrema and nucleus tractus solitarius, also expresses GLP-1 receptors. These regions process visceral sensory information and contribute to nausea responses—explaining why nausea is a common adverse effect when initiating GLP-1 receptor agonist therapy. The area postrema lies outside the blood-brain barrier, allowing circulating GLP-1 to directly access these receptors. Nausea also relates to peripheral effects, including delayed gastric emptying and vagal signalling.
Other brain regions with GLP-1 receptor expression include:
Ventral tegmental area and nucleus accumbens (reward and motivation circuits)
Amygdala (emotional processing and stress responses)
Prefrontal cortex (executive function and decision-making)
Substantia nigra (motor control, relevant to Parkinson's disease research)
This widespread distribution suggests that GLP-1 influences diverse neurological functions beyond appetite regulation. However, the blood-brain barrier limits the extent to which peripherally administered GLP-1 or GLP-1 receptor agonists can access central receptors. Effects are likely mediated via circumventricular organs (brain regions lacking a complete blood-brain barrier) and vagal afferent pathways, with limited direct penetration.
GLP-1 receptor agonists—including semaglutide, liraglutide, dulaglutide, and exenatide—are licensed in the UK for type 2 diabetes management and, in some formulations, for weight management in adults with obesity or overweight with comorbidities. Whilst their primary therapeutic targets are glycaemic control and weight reduction, their brain-mediated effects contribute significantly to clinical outcomes and adverse effect profiles.
Appetite suppression and weight loss occur predominantly through central GLP-1 receptor activation. Patients typically experience reduced hunger, earlier satiety, and decreased food cravings. These effects usually develop within the first few weeks of treatment but may diminish slightly over time as physiological adaptation occurs. The MHRA-approved indications specify appropriate patient selection criteria, and these medications should only be prescribed as part of a comprehensive weight management programme including dietary modification and increased physical activity.
Common brain-related adverse effects include nausea, which affects approximately 20–40% of patients during dose escalation. This results from GLP-1 receptor activation in the brainstem's chemoreceptor trigger zone, as well as delayed gastric emptying. Nausea is typically transient and can be minimised through gradual dose titration. Administration advice varies by product: oral semaglutide (Rybelsus) must be taken on an empty stomach with water only; exenatide immediate-release is given before meals; weekly injectables can be administered regardless of food intake. Vomiting, though less common, may occur and warrants medical review if persistent, as it can lead to dehydration and electrolyte disturbances.
Headache is reported by approximately 10–15% of patients and usually resolves within the first month of therapy. Dizziness may occur, particularly if rapid weight loss leads to postural hypotension or if patients are concurrently taking antihypertensive medications that require dose adjustment.
Important safety considerations include:
Risk of acute pancreatitis: urgent medical review needed for severe, persistent abdominal pain (with or without back pain)
Gallbladder disorders: seek medical advice for symptoms of gallstones (right upper abdominal pain, nausea)
Diabetic retinopathy complications: particularly with semaglutide in those with pre-existing retinopathy
Hypoglycaemia: risk is low unless combined with insulin or sulfonylureas, which may require dose adjustment
Reports of mood changes and suicidal ideation in patients taking GLP-1 receptor agonists are being monitored by regulatory authorities. The EMA's Pharmacovigilance Risk Assessment Committee (2024) has not established a causal association to date, but monitoring continues. Patients with a history of depression or suicidal thoughts should inform their prescriber before starting treatment, and anyone experiencing new or worsening mood symptoms should seek immediate medical attention.
When to contact your GP:
Persistent or severe nausea/vomiting preventing adequate fluid intake
Severe, persistent abdominal pain which could indicate pancreatitis
New or worsening depression, anxiety, or suicidal thoughts
Severe headaches or visual disturbances
Unexplained cognitive changes or confusion
Signs of hypoglycaemia (if taking alongside insulin or sulfonylureas)
NICE guidance recommends regular monitoring during GLP-1 receptor agonist therapy. For type 2 diabetes (NG28), treatment should continue only if there is adequate response (≥11 mmol/mol [1.0%] HbA1c reduction and ≥3% weight loss at 6 months). For weight management, specific thresholds apply: for liraglutide 3mg (Saxenda), ≥5% weight loss at 12 weeks of full dose (NICE TA664); for semaglutide 2.4mg (Wegovy), reassessment at 6 months (NICE TA875).
Patients should never adjust doses independently or source medications from unregulated suppliers, as counterfeit products pose serious health risks. All GLP-1 receptor agonist prescriptions should be initiated and monitored by healthcare professionals experienced in their use, with clear safety-netting advice provided.
Suspected side effects should be reported to the MHRA Yellow Card Scheme (yellowcard.mhra.gov.uk or the Yellow Card app).
GLP-1 receptors are found in the hypothalamus (appetite regulation), hippocampus (memory formation), brainstem (nausea responses), ventral tegmental area and nucleus accumbens (reward circuits), amygdala (emotional processing), prefrontal cortex (executive function), and substantia nigra (motor control).
Nausea occurs because GLP-1 activates receptors in the brainstem's chemoreceptor trigger zone (area postrema) and slows gastric emptying. This adverse effect affects 20–40% of patients during dose escalation but typically improves with gradual titration and usually resolves within the first few weeks of treatment.
No, GLP-1 receptor agonists are not MHRA-approved for dementia or cognitive decline. Whilst preclinical research suggests potential neuroprotective effects, clinical evidence in humans remains inconclusive, and these medications should not be used off-label for neurological conditions without specialist assessment.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.