
Tirzepatide left out of the fridge is a common concern for patients using this temperature-sensitive medication. Tirzepatide (Mounjaro) is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist prescribed for type 2 diabetes mellitus management in the UK. As a biological peptide medication, tirzepatide requires specific storage conditions to maintain its structural integrity and therapeutic efficacy. Brief exposure to room temperature does not necessarily render the medication ineffective, but understanding proper storage guidelines and knowing when to discard compromised medication is essential for safe, effective treatment. This article explains what happens when tirzepatide is left unrefrigerated, how long it remains stable at room temperature, and what steps to take if storage guidelines are breached.
Quick Answer: Tirzepatide may be kept at room temperature (not exceeding 30°C) for up to 21 days according to MHRA-approved guidance, after which it should be discarded.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereTirzepatide is a glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist used for the management of type 2 diabetes mellitus and, in some cases, weight management (marketed as Mounjaro in the UK). As a biological peptide medication, tirzepatide is sensitive to temperature fluctuations and requires specific storage conditions to maintain its structural integrity and therapeutic efficacy.
When tirzepatide is left out of the fridge, the active pharmaceutical ingredient may begin to degrade due to exposure to higher ambient temperatures. Peptide medications like tirzepatide contain complex protein structures that can potentially break down when exposed to heat, potentially reducing the medication's potency. This degradation is not always immediately visible, making it difficult for patients to determine whether their medication remains effective.
The primary concerns when tirzepatide is left unrefrigerated include:
Loss of therapeutic potency, which may result in inadequate glycaemic control
Potential changes to the solution
Increased risk of suboptimal treatment outcomes
Possible wastage of expensive medication
It is important to note that brief exposure to room temperature does not necessarily render tirzepatide completely ineffective. The manufacturer's guidance, as detailed in the Summary of Product Characteristics (SmPC), provides specific timeframes during which the medication may remain stable outside refrigeration. However, exposure beyond recommended limits can compromise the medication's quality and should be avoided. Patients who accidentally leave their tirzepatide pen out of the fridge should assess the duration of exposure and consult the product information or their healthcare provider for guidance on whether the medication can still be used safely.
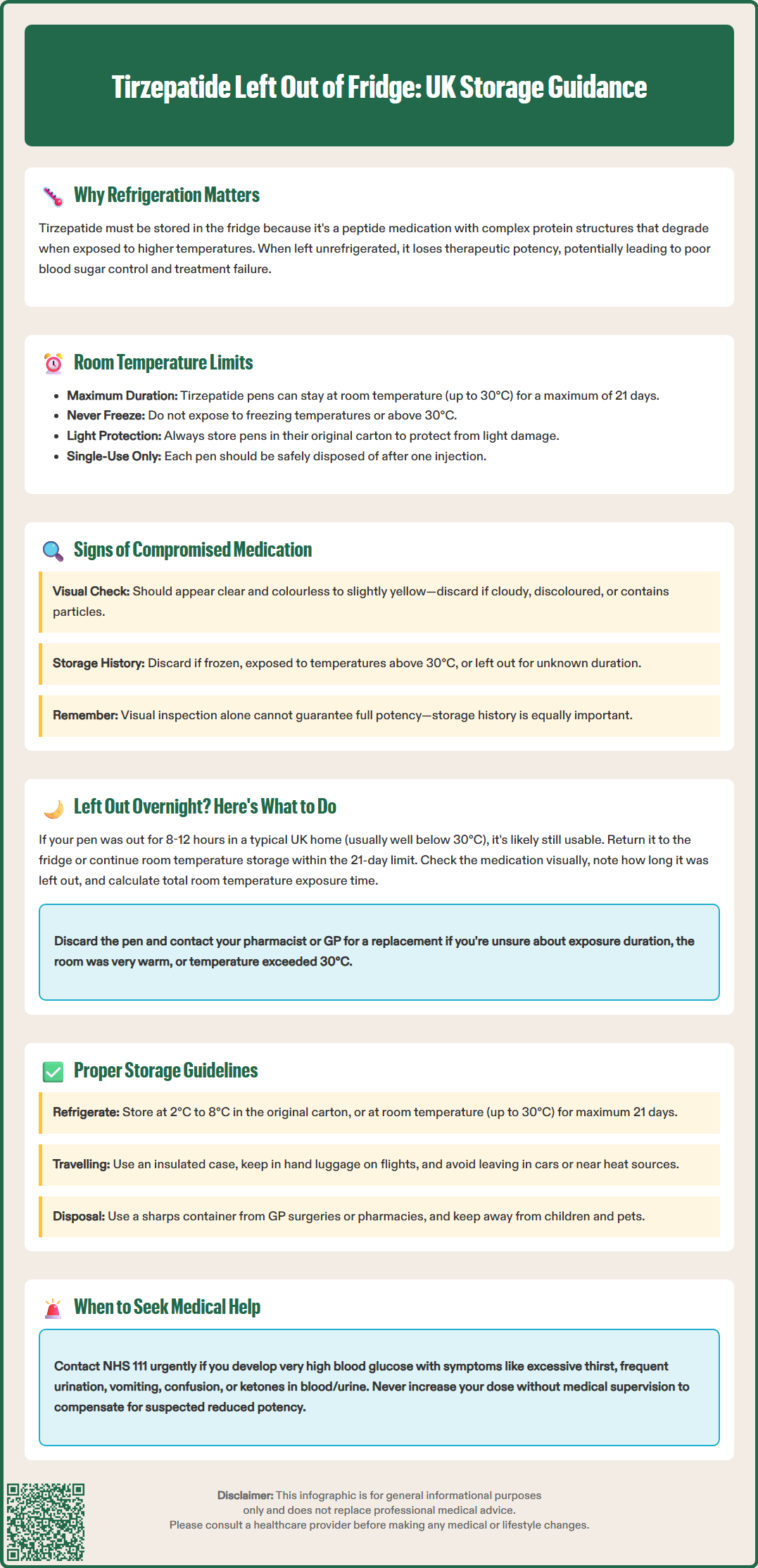
According to the manufacturer's guidance and the Summary of Product Characteristics (SmPC) approved by the Medicines and Healthcare products Regulatory Agency (MHRA), tirzepatide pens may be kept at room temperature (not exceeding 30°C) for up to 21 days. This allowance provides flexibility for patients during travel or in situations where refrigeration is temporarily unavailable. However, once this 21-day period has elapsed, the medication should be discarded, even if it appears normal.
It's important to understand that tirzepatide (Mounjaro) in the UK is supplied as single-dose, single-use pens. Each pen is used once and then disposed of safely.
Key temperature considerations include:
Room temperature is defined as not exceeding 30°C
The medication must never be frozen
Direct sunlight and heat sources should be avoided
Pens should be kept in their original carton to protect from light
If a pen has been exposed to temperatures exceeding 30°C or has been frozen, it should be discarded
It is crucial to understand that these timeframes represent maximum allowable periods under optimal conditions. If tirzepatide has been exposed to temperatures exceeding 30°C, or if the duration of room temperature storage is uncertain, the medication's stability cannot be guaranteed. In the UK climate, room temperature typically remains within acceptable limits, but during summer months or in heated environments, temperatures may exceed the recommended threshold, potentially affecting the medication's quality.
Visual inspection of your tirzepatide pen is an essential step before each injection. While some forms of degradation may not be visible to the naked eye, certain physical changes can indicate that the medication has been compromised and should not be used. According to the SmPC, tirzepatide should appear as a clear, colourless to slightly yellow solution. Any deviation from this appearance warrants caution.
Inspect your tirzepatide pen and do not use it if you observe:
Cloudiness or turbidity – the solution should be completely clear, not milky or hazy
Visible particles or foreign matter – any floating particles, sediment, or crystallisation
Discolouration – any brown, dark yellow, or other unusual colour changes
Signs of freezing – if the pen has been frozen, do not use it and dispose of it safely
Damage to the pen – cracks, leaks, or mechanical defects
Beyond visual inspection, consider the storage history of your medication. If you cannot reliably determine how long the pen has been left out of the fridge, or if it has been exposed to extreme temperatures (either freezing or heat above 30°C), it is safer to discard the pen and use a new one. The absence of visible changes does not guarantee that the medication has retained its full potency.
Patients should also be aware that using degraded or compromised tirzepatide may result in inadequate blood glucose control. If you notice unexpectedly high blood glucose readings after using a pen that may have been improperly stored, this could indicate reduced medication efficacy. In such cases, contact your GP or diabetes specialist nurse for advice. Seek urgent medical help if you experience very high blood glucose with symptoms such as increased thirst, frequent urination, dehydration, vomiting, or drowsiness/confusion, or if ketones are present in your blood or urine. Never attempt to compensate for potentially reduced potency by increasing your dose without medical supervision, as this could lead to adverse effects if you subsequently use properly stored medication.
Report any suspected side effects via the MHRA Yellow Card scheme (yellowcard.mhra.gov.uk or the Yellow Card app).
Discovering that you've accidentally left your tirzepatide pen out of the fridge overnight can be concerning, but the appropriate course of action depends on several factors. First, determine how long the medication has been at room temperature and what the ambient temperature was during this period. If the pen has been at room temperature for less than 21 days in total and the room temperature did not exceed 30°C, the medication may still be safe to use.
Immediate steps to take:
Note the date and approximate duration the pen was left out
Check the room temperature if possible (most UK homes maintain temperatures well below 30°C)
Inspect the medication visually for any signs of degradation as described in the SmPC
Calculate the total time the pen has been at room temperature
Keep the pen in its original carton to protect from light
If the pen has been left out only overnight (approximately 8-12 hours) in a typical UK home environment and has not been exposed to temperatures above 30°C, the medication is likely still suitable for use. You may return it to the refrigerator or continue storing it at room temperature, bearing in mind the 21-day limit from when it was first removed from refrigeration.
However, if you are uncertain about the duration of exposure, if the room was particularly warm (such as during a heatwave or near a radiator), or if the pen may have been exposed to temperatures above 30°C, it is advisable to err on the side of caution. Contact your community pharmacist first for product handling queries, or your GP surgery or diabetes specialist nurse if you have concerns about your glucose control. They can help you assess whether the medication should be discarded and can arrange for a replacement prescription if necessary.
If you experience very high blood glucose levels with symptoms such as increased thirst, frequent urination, dehydration, vomiting, or increasing drowsiness/confusion, or if ketones are present in your blood or urine, seek urgent medical advice from NHS 111 or your local urgent care service.
Correct storage of tirzepatide is essential to maintain its therapeutic efficacy and ensure patient safety. Understanding and following the manufacturer's storage recommendations, as approved by the MHRA in the SmPC, will help you get the most benefit from your treatment whilst minimising the risk of medication wastage.
Storage guidelines for tirzepatide pens:
Store in a refrigerator at 2°C to 8°C (typically the main body of the fridge, not the door)
Keep in the original carton to protect from light
Do not freeze; if accidentally frozen, discard the pen
May be kept at room temperature (not exceeding 30°C) for up to 21 days if needed
Check the expiry date on the carton and do not use beyond this date
Discard the pen if it has been exposed to temperatures above 30°C
When travelling with tirzepatide, use an insulated medication travel case or cool bag if you'll be away from refrigeration for extended periods, particularly during warm weather. However, ensure the medication does not come into direct contact with ice packs, as freezing will damage the medication. Never leave tirzepatide pens in a car, on a window sill, or near heaters, as temperatures can quickly exceed 30°C in these environments. If travelling by air, keep tirzepatide in your hand luggage rather than checked baggage, as cargo holds can experience freezing temperatures.
Additional safety considerations:
Always store tirzepatide out of reach of children and away from pets. Dispose of used pens in a sharps container, which can be obtained from your GP surgery or community pharmacy. Each tirzepatide (Mounjaro) pen is designed for single use only – use once and then dispose of safely. Never share your tirzepatide pen with others, even if they use the same medication, as this poses an infection risk.
If you have any questions about proper storage or notice any problems with your medication, contact your community pharmacist, diabetes specialist nurse, or GP surgery for advice. If you experience any side effects, report them via the MHRA Yellow Card scheme (yellowcard.mhra.gov.uk or the Yellow Card app).
If tirzepatide has been left out overnight at room temperature (not exceeding 30°C) and the total time at room temperature is less than 21 days, it is likely still safe to use. Inspect the solution visually for any cloudiness, discolouration, or particles before use, and contact your pharmacist or GP if uncertain.
Tirzepatide should be stored in a refrigerator at 2°C to 8°C in its original carton to protect from light. It may be kept at room temperature (not exceeding 30°C) for up to 21 days if needed, but must never be frozen.
Tirzepatide should appear as a clear, colourless to slightly yellow solution. Do not use if the solution is cloudy, contains visible particles, shows discolouration, or if the pen has been frozen or exposed to temperatures above 30°C.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.