
'Ozempic teeth' is an informal term used on social media to describe dental problems some patients report whilst taking Ozempic (semaglutide) or similar GLP-1 receptor agonist medications. These reports include tooth decay, gum disease, and tooth loss. However, no official clinical link has been established between Ozempic and direct dental damage, and UK regulators (MHRA) have not issued specific dental warnings. The reported issues may be multifactorial, potentially related to gastrointestinal side effects, dietary changes, or pre-existing conditions. Understanding these potential indirect mechanisms is important for patients and healthcare professionals managing treatment with this diabetes medication.
Quick Answer: 'Ozempic teeth' refers to anecdotal reports of dental problems attributed to Ozempic (semaglutide), though no official clinical link between the medication and direct dental damage has been established by UK regulators.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start Here'Ozempic teeth' is an informal term that has emerged on social media and in patient communities to describe dental problems some individuals have reported whilst taking Ozempic (semaglutide) or similar GLP-1 receptor agonist medications. These reports typically include complaints of tooth decay, gum disease, tooth loss, and other oral health deterioration that patients attribute to their medication. However, it is crucial to understand that there is no official clinical link established between Ozempic and direct dental damage, and as of 2023, no specific dental warnings appear in the official product information.
Ozempic is a prescription medicine licensed in the UK specifically for the treatment of type 2 diabetes mellitus. A higher-dose formulation of semaglutide (marketed as Wegovy) is separately licensed for weight management in adults with obesity or overweight with weight-related comorbidities, according to NICE criteria (TA875). The medication works by mimicking the action of glucagon-like peptide-1 (GLP-1), a naturally occurring hormone that regulates blood glucose levels, slows gastric emptying, and reduces appetite. Whilst the drug has proven efficacy in glycaemic control and weight reduction, its gastrointestinal effects may indirectly influence oral health.
The Medicines and Healthcare products Regulatory Agency (MHRA) and the European Medicines Agency (EMA) have not issued specific warnings about dental complications as a direct adverse effect of semaglutide. The reported dental issues may be multifactorial , potentially related to changes in diet, nutritional intake, gastric acid exposure from nausea and vomiting, possibly reduced saliva production (though dry mouth is not listed as a recognised side effect in the SmPC), or pre-existing dental conditions that become more apparent during treatment. Understanding the potential indirect mechanisms through which GLP-1 receptor agonists might affect oral health is important for both patients and healthcare professionals.
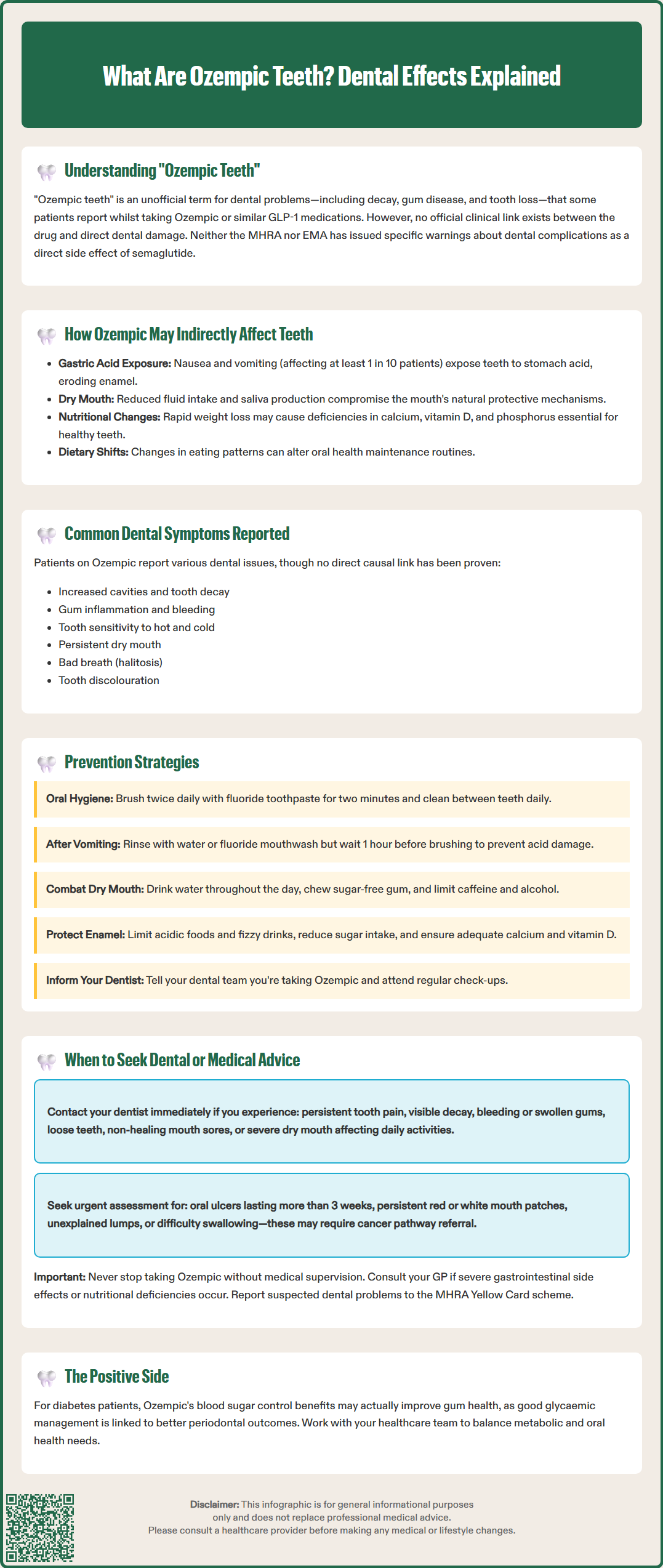
Patients taking Ozempic who report dental concerns typically describe a range of symptoms that may develop gradually over the course of treatment. It's important to note that these are anecdotal reports, and no causal relationship has been established. These symptoms may occur for various reasons while on treatment or may be coincidental. Common complaints include:
Increased tooth decay or cavities – Some individuals notice new dental caries or worsening of existing decay, particularly along the gum line or between teeth
Gum problems – Gingivitis (gum inflammation), bleeding gums, receding gums, or periodontal disease may develop or worsen
Tooth sensitivity – Heightened sensitivity to hot, cold, or sweet foods and beverages
Dry mouth (xerostomia) – A persistent feeling of oral dryness, which can compromise the mouth's natural protective mechanisms
Bad breath (halitosis) – Persistent unpleasant breath odour that does not improve with routine oral hygiene
Tooth discolouration or erosion – Changes in tooth appearance, including yellowing, thinning of enamel, or visible wear patterns
These symptoms may be indirectly related to the medication's mechanism of action and common adverse effects. According to the Ozempic SmPC, gastrointestinal side effects are very common (affecting ≥1/10 patients), including nausea, vomiting, and diarrhoea. Dyspepsia (indigestion) is also common. Repeated exposure to gastric acid can erode tooth enamel, particularly affecting the lingual (tongue-facing) surfaces of teeth. Additionally, nausea may lead to reduced fluid intake, potentially contributing to dehydration and decreased saliva production, though xerostomia is not listed as a recognised adverse reaction in the product information.
Significant weight loss, whilst therapeutically beneficial, could theoretically result in nutritional deficiencies if dietary intake is severely restricted or unbalanced. Inadequate calcium, vitamin D, phosphorus, and other minerals essential for dental health could potentially compromise tooth and bone integrity, though this mechanism remains hypothetical. Furthermore, changes in eating patterns—such as increased consumption of acidic beverages, frequent snacking on soft or sugary foods due to nausea, or poor appetite leading to neglect of oral hygiene—may all contribute to dental deterioration.
It's worth noting that good glycaemic control in diabetes is associated with better periodontal outcomes, so the benefits of improved blood glucose management with Ozempic may positively impact gum health in some patients with type 2 diabetes.
Proactive oral hygiene and lifestyle modifications are essential for patients taking Ozempic to minimise the risk of dental complications. The following evidence-based strategies can help maintain optimal oral health during treatment:
Maintain rigorous oral hygiene:
Brush teeth at least twice daily with fluoride toothpaste (1450 ppm fluoride), using proper technique for two minutes
Clean between teeth daily using interdental brushes (preferred where spaces allow) or floss to remove plaque and food debris
Consider using an alcohol-free fluoride mouthwash to protect teeth; antimicrobial mouthwashes should only be used short-term and on dental advice
After vomiting, rinse with water or a fluoride mouthwash and wait at least 1 hour before brushing to avoid spreading acid across tooth surfaces
Address dry mouth proactively:
Maintain adequate hydration by sipping water throughout the day
Use sugar-free gum or lozenges to stimulate saliva production
Consider saliva substitutes or oral moisturising gels if xerostomia is persistent
Limit caffeine and alcohol, which can exacerbate dry mouth
Manage gastrointestinal side effects:
Take Ozempic as prescribed and follow your healthcare provider's advice on managing nausea
Eat smaller, more frequent meals to reduce the likelihood of vomiting
Avoid lying down immediately after eating to minimise acid reflux
If experiencing frequent vomiting or reflux, discuss with your GP whether acid-suppressing medication might be appropriate
Optimise nutrition:
Ensure adequate intake of calcium, vitamin D, and other nutrients essential for dental and bone health
Limit acidic foods and beverages (citrus fruits, fizzy drinks, fruit juices)
Reduce sugar consumption to decrease the risk of dental caries
Consider consultation with a dietitian to ensure nutritional adequacy during weight loss
Regular dental monitoring:
Inform your dentist that you are taking Ozempic
Attend routine dental check-ups at intervals recommended by your dentist based on your individual risk (as per NICE guidance)
Discuss with your dentist whether high-fluoride toothpaste (2800-5000 ppm) might be beneficial if you're at high risk of dental caries
Address any dental concerns promptly rather than waiting for scheduled appointments
Patients taking Ozempic should be vigilant about their oral health and seek professional advice when specific warning signs emerge. Contact your dentist promptly if you experience:
Persistent tooth pain or sensitivity that does not resolve with over-the-counter treatments
Visible tooth decay, discolouration, or structural changes to teeth
Bleeding, swollen, or receding gums that persist despite improved oral hygiene
Loose teeth or changes in bite alignment
Persistent bad breath unresponsive to oral hygiene measures
Mouth sores, ulcers, or unusual lesions that do not heal within two weeks
Severe or persistent dry mouth affecting eating, speaking, or quality of life
These symptoms warrant professional dental assessment to prevent progression of dental disease and to implement appropriate treatment. Early intervention can often prevent more serious complications such as tooth loss or systemic infection.
Particularly concerning symptoms that require urgent assessment include oral ulcers that don't heal after 3 weeks, persistent red or white patches in the mouth, unexplained lumps, or persistent hoarseness/difficulty swallowing, as these may require referral via the suspected cancer pathway (NICE NG12).
Consult your GP or prescribing clinician if:
Gastrointestinal side effects (nausea, vomiting, reflux) are severe, persistent, or worsening, as these may indirectly affect oral health
You are unable to maintain adequate nutrition or hydration due to medication side effects
You develop signs of nutritional deficiency (fatigue, weakness, bone pain)
Dental problems are significantly impacting your quality of life or ability to continue treatment
Your healthcare provider can assess whether dose adjustment, additional supportive medications, or alternative treatment options might be appropriate. According to NICE guidance (NG28 for type 2 diabetes; TA875 for weight management), treatment should be individualised, with regular review of efficacy and tolerability. If dental complications are attributed to Ozempic, your clinician will weigh the benefits of continued treatment against potential risks and may consider alternative therapeutic approaches.
Do not discontinue Ozempic without medical advice, as abrupt cessation may lead to deterioration in glycaemic control or weight regain. A collaborative approach involving your GP, dentist, and diabetes specialist nurse (where applicable) ensures comprehensive care that addresses both your metabolic health and oral wellbeing.
If you suspect your dental problems may be related to Ozempic, you can report possible side effects via the MHRA Yellow Card scheme at yellowcard.mhra.gov.uk, through the Yellow Card app, or by asking your healthcare professional to report on your behalf.
No, there is no official clinical link established between Ozempic and direct dental damage. The MHRA and EMA have not issued specific warnings about dental complications as a recognised adverse effect of semaglutide.
Anecdotal reports include increased tooth decay, gum inflammation, tooth sensitivity, dry mouth, bad breath, and tooth discolouration. These may be indirectly related to gastrointestinal side effects such as nausea and vomiting rather than direct medication effects.
Maintain rigorous oral hygiene with fluoride toothpaste, stay well hydrated, manage gastrointestinal side effects, ensure adequate nutrition, and attend regular dental check-ups. Inform your dentist that you are taking Ozempic and address any concerns promptly.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.