Many people with epilepsy who are overweight or living with obesity wonder whether Wegovy (semaglutide) is a safe option for weight management. Epilepsy is not listed as a contraindication to Wegovy, and the medication does not directly affect seizure thresholds or interact significantly with most anti-epileptic drugs. However, individual circumstances vary, and factors such as gastrointestinal side effects, medication absorption, and overall seizure control require careful consideration. This article explores the safety of Wegovy for people with epilepsy, potential risks, and the importance of consulting your healthcare team before starting treatment.
Quick Answer: Epilepsy is not a contraindication to Wegovy, and people with epilepsy may use semaglutide for weight management following individual medical assessment.
Wegovy (semaglutide 2.4 mg) is a prescription medicine licensed in the UK for weight management in adults with obesity or those who are overweight with at least one weight-related comorbidity. It belongs to a class of medications called glucagon-like peptide-1 (GLP-1) receptor agonists, which were originally developed for type 2 diabetes management.
The medication works by mimicking a naturally occurring hormone called GLP-1, which is released in the gut after eating. Semaglutide acts on specific receptors in the brain that regulate appetite and food intake, helping patients feel fuller for longer periods and reducing overall calorie consumption. Additionally, it slows gastric emptying, which contributes to prolonged satiety after meals.
Wegovy is administered as a once-weekly subcutaneous injection, typically into the abdomen, thigh, or upper arm. The treatment follows a gradual dose escalation schedule over 16 weeks (0.25→0.5→1.0→1.7→2.4 mg weekly), with the option to delay escalation or maintain at 1.7 mg if the higher dose is not tolerated. This approach helps minimise gastrointestinal side effects and improve tolerability.
According to NICE guidance (TA875), Wegovy should be used alongside a reduced-calorie diet and increased physical activity as part of a comprehensive weight management programme. In England, access is typically through specialist weight management services (tier 3 or 4), with specific BMI thresholds for eligibility, and treatment is usually limited to up to 2 years.
Clinical trials have demonstrated that patients using Wegovy can achieve significant weight loss—typically around 15% of their initial body weight over 68 weeks in people without diabetes, and approximately 10% in those with type 2 diabetes. Individual results vary considerably. The medication is particularly beneficial for individuals with obesity-related conditions such as hypertension, dyslipidaemia, obstructive sleep apnoea, or cardiovascular disease. However, like all medications, Wegovy is not suitable for everyone and requires careful assessment of individual medical history, including any neurological conditions such as epilepsy.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereEpilepsy is a chronic neurological condition characterised by recurrent, unprovoked seizures resulting from abnormal electrical activity in the brain. Management typically involves long-term treatment with anti-epileptic drugs (AEDs) or anti-seizure medications (ASMs), which work through various mechanisms to stabilise neuronal activity and prevent seizure occurrence.
People with epilepsy must exercise particular caution when starting new medications, as drug interactions can potentially affect seizure control in several ways. Some medications may lower the seizure threshold, making seizures more likely to occur. Others may interact with anti-epileptic drugs by altering their metabolism through the liver's cytochrome P450 enzyme system, potentially reducing their effectiveness or increasing side effects.
Common anti-epileptic medications include sodium valproate, lamotrigine, levetiracetam, carbamazepine, and phenytoin, each with distinct interaction profiles. For instance, enzyme-inducing AEDs like carbamazepine and phenytoin can accelerate the metabolism of other drugs, whilst some medications can increase or decrease AED blood levels, affecting seizure control.
Regarding Wegovy (semaglutide), it is important to note that it does not significantly affect cytochrome P450 enzymes, and clinically relevant pharmacokinetic interactions with common ASMs are not expected. However, semaglutide's effect on delaying gastric emptying may affect the absorption of oral medicines with a narrow therapeutic index, which could include some anti-epileptic drugs. This is particularly relevant if the timing of peak concentration (Tmax) is important for efficacy or safety.
Beyond direct pharmacological interactions, other factors warrant consideration. Metabolic changes, such as significant weight loss or alterations in blood glucose levels, can potentially influence seizure patterns in some individuals. Additionally, gastrointestinal effects that cause vomiting or severe nausea might affect the absorption of oral anti-epileptic medications, potentially compromising seizure control. Patients should report persistent vomiting or poor oral intake promptly to their healthcare team.
Anyone with epilepsy considering a new medication should discuss potential interactions with their neurologist or epilepsy specialist nurse. Maintaining a seizure diary and monitoring any changes in seizure frequency or pattern when starting new treatments is essential for safe medication management.
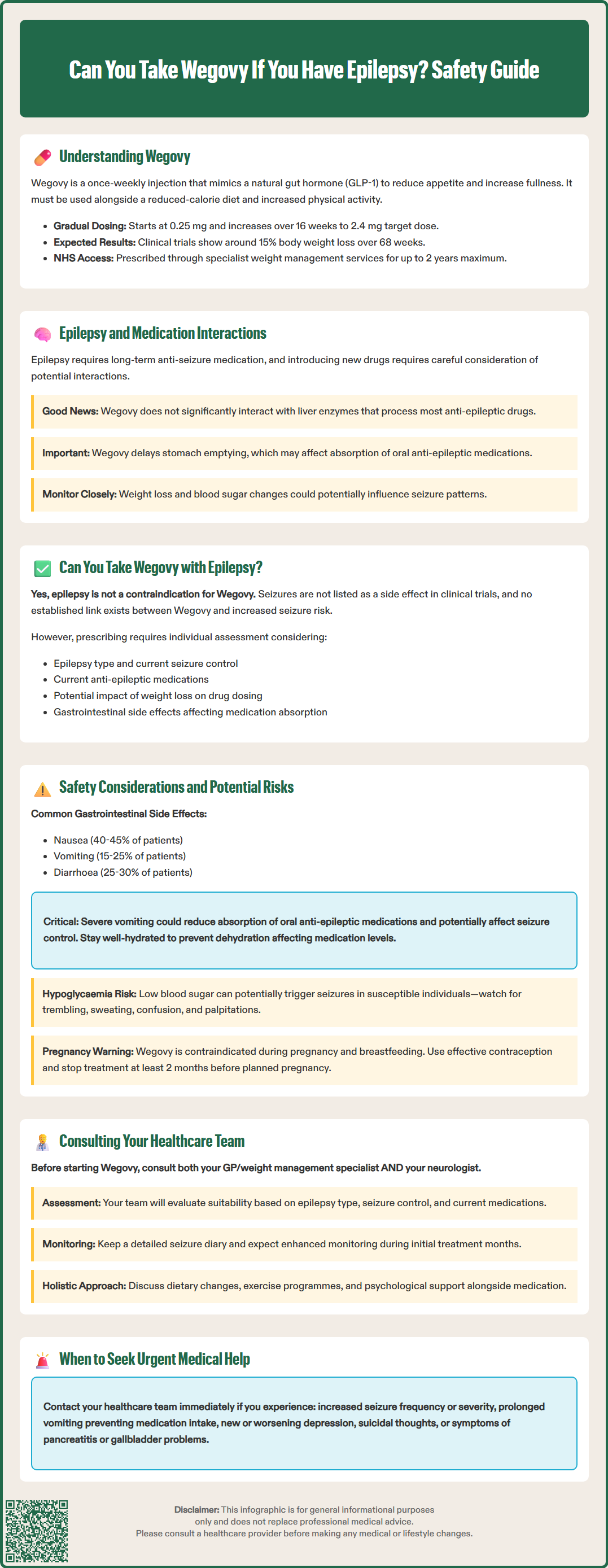
Epilepsy is not listed as a contraindication to Wegovy in the Summary of Product Characteristics (SmPC) approved by the Medicines and Healthcare products Regulatory Agency (MHRA). This means that having epilepsy does not automatically prevent you from using semaglutide for weight management. Importantly, seizures are not listed as an adverse reaction in the SmPC, and no signal of lowered seizure threshold has been identified in clinical trials or post-marketing surveillance to date.
Currently, there is no official established link between Wegovy and increased seizure risk or worsening of epilepsy control in clinical literature. The medication's primary mechanism of action—targeting GLP-1 receptors to regulate appetite—does not directly interfere with neuronal excitability or seizure thresholds in the way that some other medications might.
That said, individual circumstances vary considerably. The decision to prescribe Wegovy to someone with epilepsy requires careful consideration of several factors, including the type and severity of epilepsy, current seizure control, the specific anti-epileptic medications being taken, and overall health status. Some considerations include whether significant weight loss might affect medication dosing requirements or whether gastrointestinal side effects could impact absorption of oral AEDs.
Some research suggests that obesity and metabolic syndrome may be associated with increased inflammation and other factors that could potentially influence neurological health, including seizure control in some individuals. However, the relationship between weight loss and seizure control is complex and not fully understood, with limited high-quality evidence specifically addressing this relationship.
The key message is that Wegovy may be prescribed to people with epilepsy, but this decision must be made on an individual basis following thorough medical assessment and discussion with appropriate specialists.
When considering Wegovy for someone with epilepsy, several safety considerations require careful evaluation. Whilst there is no direct contraindication, understanding potential indirect effects is essential for safe prescribing and monitoring.
Gastrointestinal side effects are the most common adverse reactions to Wegovy, affecting a significant proportion of users, particularly during dose escalation. According to the SmPC and STEP clinical trials, these include:
Nausea (affecting approximately 40-45% of patients)
Vomiting (approximately 15-25% of patients)
Diarrhoea (affecting approximately 25-30% of users)
Constipation (approximately 20-25% of patients)
Abdominal pain and bloating
Gastro-oesophageal reflux
For individuals taking oral anti-epileptic medications, severe or persistent vomiting could theoretically reduce drug absorption, potentially affecting seizure control. If you experience significant gastrointestinal symptoms, it is important to contact your healthcare team promptly, particularly if you notice any changes in seizure patterns.
Hypoglycaemia (low blood sugar) is another consideration, particularly for people with epilepsy who also have diabetes and take insulin or sulphonylureas. Whilst Wegovy alone rarely causes hypoglycaemia, low blood sugar can potentially trigger seizures in susceptible individuals. Symptoms include trembling, sweating, confusion, and palpitations.
Other recognised adverse effects of Wegovy include:
Fatigue and dizziness (which might be confused with seizure-related symptoms)
Increased heart rate (typically modest, around 1–4 beats per minute)
Gallbladder problems (cholelithiasis)
Pancreatitis (rare but serious)
Important additional safety considerations include:
Pregnancy and breastfeeding: Wegovy is contraindicated during pregnancy and breastfeeding. Women of childbearing potential should use effective contraception and stop treatment at least 2 months before a planned pregnancy.
Diabetic retinopathy: People with type 2 diabetes and pre-existing retinopathy should be monitored closely, as rapid improvement in glucose control has been associated with temporary worsening of retinopathy.
Mental health: The MHRA is currently reviewing reports of suicidal thoughts and self-harming behaviours with GLP-1 receptor agonists. Seek medical advice promptly for new or worsening depression or suicidal thoughts.
Dehydration and renal function: Dehydration resulting from gastrointestinal side effects requires attention, as it can affect overall health, medication levels, and potentially lead to acute kidney injury, especially in those with pre-existing renal impairment. Maintaining adequate fluid intake is essential, particularly during the initial weeks of treatment.
Regular monitoring and open communication with your healthcare team enable early identification of any concerns and appropriate management adjustments if needed.
If you have epilepsy and are considering Wegovy for weight management, comprehensive consultation with your healthcare team is essential before starting treatment. This collaborative approach ensures that all aspects of your medical history are considered and appropriate monitoring arrangements are established.
In England, access to Wegovy on the NHS is typically through specialist tier 3 or 4 weight management services, in line with NICE guidance (TA875). Treatment is usually limited to up to 2 years. Your eligibility will depend on specific BMI thresholds and weight-related comorbidities.
Your GP or weight management specialist will conduct a thorough assessment, including your current weight, body mass index (BMI), weight-related comorbidities, previous weight loss attempts, and overall suitability for GLP-1 receptor agonist therapy. They will review your complete medication list to identify any potential interactions or concerns.
Your neurologist or epilepsy specialist should be informed about any proposed new medications. They can provide specific guidance regarding your epilepsy type, current seizure control, and whether any particular concerns exist with your anti-epileptic medication regimen. This specialist input is particularly valuable if your epilepsy is difficult to control or if you take multiple AEDs.
Key questions to discuss with your healthcare team include:
Is Wegovy appropriate given my specific type of epilepsy and current control?
Could gastrointestinal side effects affect my anti-epileptic medication absorption?
What monitoring arrangements should be in place?
What symptoms should prompt me to seek urgent medical advice?
Are there alternative weight management approaches that might be more suitable?
Your healthcare team may recommend enhanced monitoring during the initial months of treatment, including regular review of seizure frequency and pattern. Maintaining an accurate seizure diary becomes particularly important when starting any new medication.
When to seek urgent medical advice:
Call 999 if a seizure lasts more than 5 minutes, there are repeated seizures without recovery between them, or serious injury occurs
Any increase in seizure frequency or severity
Prolonged vomiting preventing medication intake
Severe, persistent abdominal pain (especially if radiating to the back, with or without vomiting)
Signs of dehydration
Symptoms of hypoglycaemia (if diabetic)
Any unexplained neurological symptoms
New or worsening depression or thoughts of self-harm
Remember that weight management is multifaceted, and medication is just one component. Your healthcare team can provide guidance on dietary modifications, physical activity programmes, and psychological support that complement pharmacological treatment. For some individuals with epilepsy, alternative approaches to weight management may be more appropriate, and this should be discussed openly with your medical team to identify the safest and most effective strategy for your individual circumstances.
Wegovy (semaglutide) does not significantly affect cytochrome P450 enzymes, so clinically relevant pharmacokinetic interactions with common anti-seizure medications are not expected. However, delayed gastric emptying may affect absorption of oral medicines with narrow therapeutic indices, so individual assessment is important.
Seizures are not listed as an adverse reaction to Wegovy in the Summary of Product Characteristics, and no signal of lowered seizure threshold has been identified in clinical trials or post-marketing surveillance to date.
Yes, you should inform your neurologist or epilepsy specialist before starting Wegovy. They can provide specific guidance regarding your epilepsy type, current seizure control, and whether any particular concerns exist with your anti-epileptic medication regimen.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.