
Ozempic (semaglutide) is a glucagon-like peptide-1 (GLP-1) receptor agonist licensed in the UK for treating type 2 diabetes mellitus in adults. Emerging evidence suggests potential renal protective effects in patients with chronic kidney disease (CKD), a common complication of diabetes. The landmark FLOW trial demonstrated that semaglutide significantly reduced kidney disease progression in people with type 2 diabetes and CKD. Whilst Ozempic can be used safely in patients with kidney impairment without dose adjustment, careful monitoring and patient education are essential. This article examines the use of Ozempic in kidney disease, including safety considerations, monitoring requirements, and alternative treatment options.
Quick Answer: Ozempic (semaglutide) can be used in patients with chronic kidney disease without dose adjustment, and recent evidence suggests it may reduce kidney disease progression in people with type 2 diabetes.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereOzempic (semaglutide) is a prescription medicine licensed in the UK for the treatment of type 2 diabetes mellitus in adults. It belongs to a class of medications called glucagon-like peptide-1 (GLP-1) receptor agonists, which work by mimicking the action of a naturally occurring hormone that helps regulate blood glucose levels.
The mechanism of action involves several complementary pathways. Ozempic stimulates insulin secretion from pancreatic beta cells in a glucose-dependent manner, meaning it only promotes insulin release when blood sugar levels are elevated. This reduces the risk of hypoglycaemia compared to some other diabetes medications. Additionally, it suppresses the release of glucagon (a hormone that raises blood glucose) and slows gastric emptying, which helps moderate post-meal glucose spikes.
Ozempic is administered as a once-weekly subcutaneous injection using a pre-filled pen device. The usual starting dose is 0.25 mg once weekly for four weeks, which is a titration dose intended to improve gastrointestinal tolerability rather than provide glycaemic control. This is then increased to 0.5 mg weekly, with the option to escalate to 1 mg weekly if additional glycaemic control is needed. In some cases, a maximum dose of 2 mg weekly may be prescribed.
Beyond glucose control, clinical trials have demonstrated that semaglutide offers cardiovascular benefits in people with type 2 diabetes and established cardiovascular disease. The SUSTAIN-6 trial showed a significant reduction in major adverse cardiovascular events. More recently, evidence has emerged regarding potential renal protective effects in patients with coexisting kidney disease—a common complication of diabetes. However, it is important to note that Ozempic is currently licensed only for glycaemic control in type 2 diabetes, not specifically for kidney protection.
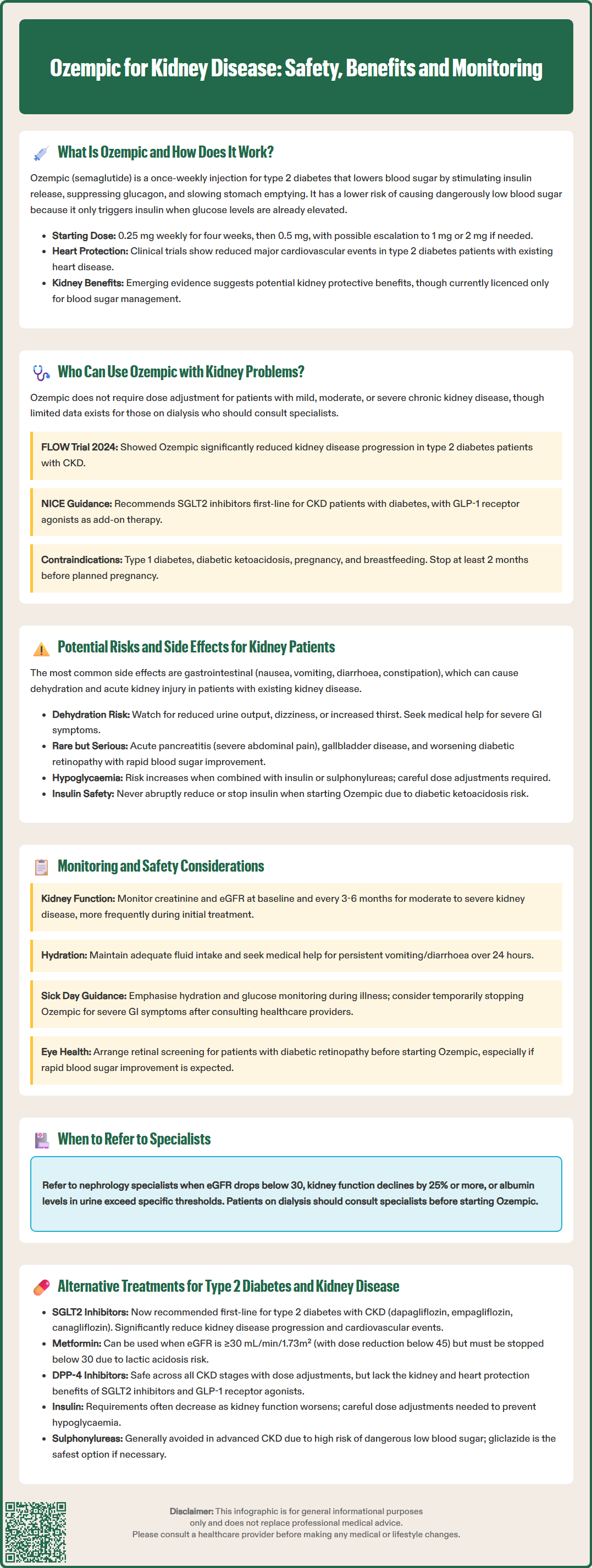
Ozempic can be used in patients with chronic kidney disease (CKD), including those with moderate to severe renal impairment. Unlike some diabetes medications that require dose adjustment or are contraindicated in kidney disease, semaglutide does not require dose modification based on renal function.
According to the Summary of Product Characteristics (SmPC) approved by the MHRA, no dose adjustment is necessary for patients with mild, moderate, or severe renal impairment. However, there is limited clinical experience in patients with end-stage kidney disease requiring dialysis, so caution and specialist input are advised in this population.
The landmark FLOW trial (published in 2024 in the New England Journal of Medicine) specifically examined semaglutide in patients with type 2 diabetes and chronic kidney disease. This study demonstrated that Ozempic significantly reduced the risk of kidney disease progression, including the composite outcome of sustained decline in eGFR, progression to end-stage kidney disease, or death from kidney or cardiovascular causes. While these findings are promising, it's important to note that this renoprotective effect is not yet reflected in the UK product licence or NICE guidance.
NICE guidance on the management of type 2 diabetes (NG28) recommends considering GLP-1 receptor agonists for patients who have not achieved adequate glycaemic control with other therapies, particularly when weight loss would be beneficial. For patients with CKD, NICE guideline NG203 recommends SGLT2 inhibitors as first-line therapy where suitable, with GLP-1 receptor agonists considered as add-on therapy. The choice of diabetes medication should be made in consultation with diabetes specialists or nephrologists, taking into account individual patient factors, comorbidities, and treatment goals.
Ozempic should not be used in type 1 diabetes or diabetic ketoacidosis. It should be avoided in pregnancy and during breastfeeding, and women of childbearing potential should use effective contraception. The SmPC advises discontinuing semaglutide at least 2 months before a planned pregnancy.
While Ozempic is generally well-tolerated in patients with kidney disease, certain adverse effects warrant particular attention in this population. The most common side effects are gastrointestinal, including nausea, vomiting, diarrhoea, and constipation. These typically occur during dose escalation and often diminish over time. However, in patients with existing kidney impairment, severe or persistent vomiting and diarrhoea can lead to dehydration and acute kidney injury (AKI).
Patients with CKD may be more susceptible to volume depletion due to reduced renal reserve. It is therefore essential that individuals experiencing significant gastrointestinal symptoms maintain adequate fluid intake and seek medical advice promptly. Healthcare professionals should counsel patients about recognising signs of dehydration, such as reduced urine output, dizziness, or increased thirst.
There have been post-marketing reports of acute kidney injury in patients treated with GLP-1 receptor agonists, sometimes requiring haemodialysis. Most cases occurred in patients who experienced nausea, vomiting, or diarrhoea leading to volume depletion. Patients with pre-existing kidney disease may be at higher risk, emphasising the importance of appropriate patient education and monitoring.
Other relevant side effects include:
Diabetic retinopathy complications: Rapid improvement in glycaemic control has been associated with temporary worsening of diabetic retinopathy. Patients with pre-existing retinopathy should be monitored closely, particularly when HbA1c is falling quickly.
Pancreatitis: Although rare, acute pancreatitis has been reported. Patients should be advised to seek immediate medical attention if they experience severe, persistent abdominal pain.
Gallbladder disease: Cholelithiasis and cholecystitis have been reported with GLP-1 receptor agonists. Patients should seek medical review if they experience persistent right upper quadrant pain, fever, or jaundice.
Hypoglycaemia: The risk is low when Ozempic is used alone but increases when combined with insulin or sulphonylureas. Dose adjustment of these concomitant medications may be necessary.
The MHRA has issued a Drug Safety Update warning not to reduce or stop insulin abruptly when starting semaglutide, as this may increase the risk of diabetic ketoacidosis. Insulin doses should be titrated cautiously under medical supervision.
Patients should be advised to contact their GP or diabetes team if they experience persistent vomiting, severe abdominal pain, signs of dehydration, or any other concerning symptoms. Suspected adverse reactions to Ozempic should be reported via the MHRA Yellow Card scheme.
Appropriate monitoring is essential when prescribing Ozempic to patients with kidney disease to maximise safety and therapeutic benefit. Baseline assessment should include measurement of renal function (serum creatinine and eGFR), HbA1c, blood pressure, and urinary albumin-to-creatinine ratio (ACR). A comprehensive medication review should identify any drugs that may require dose adjustment or pose additional renal risk.
Regular monitoring of renal function is recommended, particularly during the initial months of treatment and during dose escalation. The frequency should be individualised based on the severity of kidney disease and other risk factors. For patients with moderate to severe CKD (eGFR <60 mL/min/1.73m²), renal function should typically be checked at least every 3–6 months, or more frequently if clinically indicated.
Patients should be educated about the importance of maintaining adequate hydration, especially during intercurrent illness or when experiencing gastrointestinal side effects. Clear written information should be provided about when to seek medical advice, including:
Persistent vomiting or diarrhoea lasting more than 24 hours
Signs of dehydration (reduced urine output, dizziness, dry mouth)
Severe abdominal pain
Symptoms of hypoglycaemia if taking insulin or sulphonylureas
Sick day guidance is particularly important for patients with CKD. During acute illness, patients should prioritise hydration and glucose monitoring. They should seek clinical advice if unable to maintain oral intake or experiencing severe gastrointestinal symptoms. Temporary withholding of semaglutide may be considered in cases of severe vomiting or diarrhoea, but this decision should be made in consultation with healthcare professionals rather than by patients independently.
Patients with pre-existing diabetic retinopathy should have appropriate retinal screening when initiating or intensifying treatment with Ozempic, particularly if rapid improvements in HbA1c are anticipated.
Referral to nephrology services should be considered in line with NICE guideline NG203 criteria, including:
eGFR <30 mL/min/1.73m²
Sustained decline in eGFR of ≥25% and a change in CKD category
ACR ≥70 mg/mmol (unless known to be caused by diabetes and already appropriately treated)
ACR ≥30 mg/mmol with haematuria
Refractory hypertension
For patients with progressive kidney disease, ongoing collaboration between primary care, diabetes specialists, and nephrology services ensures optimal management. Regular review of the overall treatment plan, including blood pressure control, use of renin-angiotensin system inhibitors, and management of other cardiovascular risk factors, is essential for comprehensive kidney protection.
Several alternative medication classes are available for managing type 2 diabetes in patients with chronic kidney disease, each with distinct benefits and limitations. The choice depends on individual patient factors, degree of renal impairment, cardiovascular risk, and treatment goals.
Sodium-glucose co-transporter-2 (SGLT2) inhibitors such as dapagliflozin, empagliflozin, and canagliflozin have emerged as important agents for kidney protection. Multiple trials (DAPA-CKD, EMPA-KIDNEY, CREDENCE) have demonstrated significant reductions in CKD progression and cardiovascular events. NICE recommends SGLT2 inhibitors for adults with type 2 diabetes and CKD (NG203), with specific guidance in technology appraisals TA775 (dapagliflozin) and TA942 (empagliflozin). eGFR thresholds for initiation vary by agent and indication; for example, dapagliflozin can be initiated for CKD at eGFR ≥25 mL/min/1.73m² and continued below this threshold. These medications can be used alongside GLP-1 receptor agonists for additive renal and cardiovascular benefits.
Metformin remains a first-line option when tolerated and appropriate. Current guidance permits its use in patients with eGFR ≥30 mL/min/1.73m², with dose reduction required below 45 mL/min/1.73m². It should be discontinued if eGFR falls below 30 mL/min/1.73m² due to increased risk of lactic acidosis.
Dipeptidyl peptidase-4 (DPP-4) inhibitors such as linagliptin, sitagliptin, and saxagliptin can be used across all stages of CKD, though most require dose adjustment in moderate to severe impairment. They are generally well-tolerated with low hypoglycaemia risk but lack the cardiovascular and renal benefits demonstrated with GLP-1 receptor agonists and SGLT2 inhibitors.
Pioglitazone (a thiazolidinedione) can be used in CKD without dose adjustment but should be used with caution due to the risk of fluid retention and heart failure, particularly in advanced kidney disease.
Insulin therapy may be necessary for patients with advanced CKD or inadequate glycaemic control with oral agents. Insulin requirements may decrease as renal function declines due to reduced renal insulin clearance, necessitating careful dose titration and glucose monitoring.
Sulphonylureas are generally avoided in advanced CKD due to accumulation of active metabolites and increased hypoglycaemia risk. If used, gliclazide is preferred as it has a lower risk profile in renal impairment.
The optimal approach often involves combination therapy tailored to individual needs, with regular review and adjustment as kidney function changes. Referral to specialist diabetes or nephrology services should be considered for complex cases or progressive kidney disease.
No, Ozempic does not require dose adjustment in patients with mild, moderate, or severe renal impairment. However, there is limited clinical experience in end-stage kidney disease requiring dialysis, so specialist input is advised in this population.
The FLOW trial demonstrated that semaglutide significantly reduced kidney disease progression in patients with type 2 diabetes and chronic kidney disease. However, this renoprotective effect is not yet reflected in UK product licensing, and Ozempic is currently approved only for glycaemic control.
The main risks include gastrointestinal side effects (nausea, vomiting, diarrhoea) that can lead to dehydration and acute kidney injury in patients with existing kidney impairment. Patients should maintain adequate hydration and seek medical advice if experiencing persistent symptoms.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.