
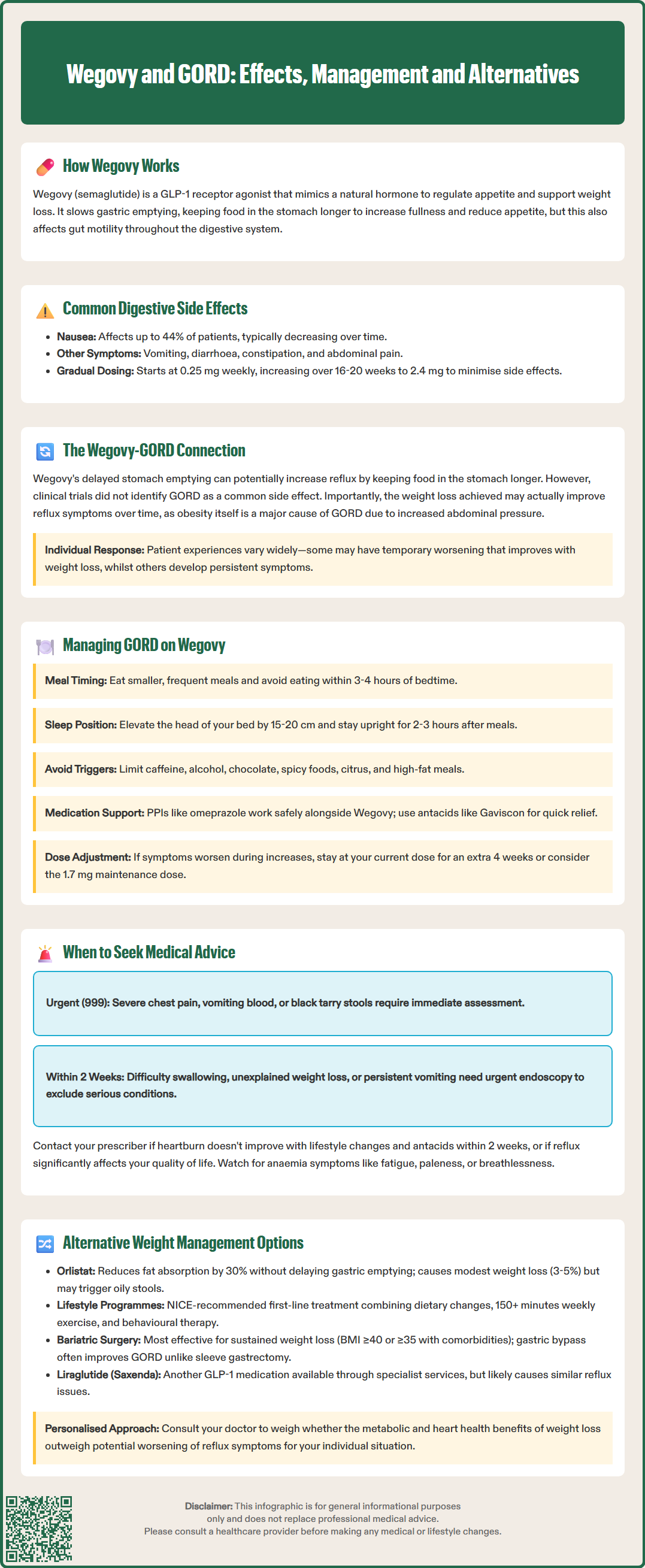
Wegovy (semaglutide 2.4 mg) is a GLP-1 receptor agonist licensed for chronic weight management in adults with obesity. Whilst highly effective for weight loss, its mechanism of slowing gastric emptying raises questions about potential effects on gastro-oesophageal reflux disease (GORD). Understanding the relationship between Wegovy and GORD is essential for patients and clinicians, as obesity itself is a major risk factor for reflux. This article examines how Wegovy may influence GORD symptoms, evidence-based management strategies, and when to seek medical advice. We also explore alternative weight management options for those with pre-existing severe reflux.
Quick Answer: Wegovy may initially worsen GORD symptoms due to delayed gastric emptying, but weight loss often improves reflux over time in many patients.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereWegovy (semaglutide 2.4 mg) is a glucagon-like peptide-1 (GLP-1) receptor agonist licensed by the MHRA for chronic weight management in adults with obesity or overweight with weight-related comorbidities. It works by mimicking the naturally occurring hormone GLP-1, which regulates appetite and food intake through actions on the brain's satiety centres.
The mechanism of action of Wegovy directly affects the digestive system in several ways. GLP-1 receptor agonists slow gastric emptying, meaning food remains in the stomach for longer periods before moving into the small intestine. This delayed gastric emptying contributes to increased feelings of fullness and reduced appetite, which supports weight loss. Additionally, semaglutide affects gut motility throughout the gastrointestinal tract.
Common gastrointestinal side effects reported with Wegovy include nausea (affecting up to 44% of patients), vomiting, diarrhoea, constipation, and abdominal pain. These effects are typically most pronounced during the initial titration phase and often diminish over time as the body adapts to the medication. The gastrointestinal tolerability profile is dose-dependent, which is why Wegovy is initiated at a low dose (0.25 mg weekly) and gradually increased over 16–20 weeks to the maintenance dose of 2.4 mg weekly.
Importantly, the MHRA SmPC notes that Wegovy is not recommended in patients with severe gastrointestinal disease, including severe gastroparesis.
Understanding how Wegovy affects digestive function is essential for patients and healthcare professionals, particularly when considering its use in individuals with pre-existing gastrointestinal conditions such as gastro-oesophageal reflux disease (GORD). The physiological changes induced by GLP-1 receptor agonists may have implications for reflux symptoms, necessitating careful consideration and monitoring.
Gastro-oesophageal reflux disease (GORD) occurs when stomach acid frequently flows back into the oesophagus, causing symptoms such as heartburn, regurgitation, and chest discomfort. The relationship between Wegovy and GORD is complex and not fully established in clinical literature, though several mechanisms warrant consideration.
The delayed gastric emptying caused by semaglutide theoretically could influence reflux symptoms in either direction. On one hand, slower gastric emptying means food remains in the stomach longer, potentially increasing intra-gastric pressure and the likelihood of reflux episodes, particularly when lying down or bending over. Some patients report worsening heartburn or new-onset reflux symptoms after starting GLP-1 receptor agonists.
Clinical trial data from the STEP (Semaglutide Treatment Effect in People with obesity) programme reported gastrointestinal adverse events but did not specifically highlight GORD as a common side effect. The Wegovy SmPC lists dyspepsia as a common adverse reaction (affecting 1-10% of patients). It is important to note that obesity itself is a significant risk factor for GORD, as excess abdominal adiposity increases intra-abdominal pressure and promotes reflux. Therefore, the weight loss achieved with Wegovy may actually improve GORD symptoms over time in many patients, as supported by studies showing improvement in reflux symptoms following weight reduction.
Individual variation in response is considerable. Some patients with pre-existing GORD may find their symptoms worsen initially but improve as weight loss progresses, whilst others may experience persistent or new reflux symptoms requiring management adjustments. There is currently no official contraindication to using Wegovy in patients with GORD, but careful assessment and monitoring are advisable.
For patients experiencing GORD symptoms whilst taking Wegovy, several evidence-based management strategies can help minimise discomfort and support treatment continuation. A multifaceted approach addressing both lifestyle modifications and pharmacological interventions typically yields the best outcomes.
Lifestyle and dietary modifications form the cornerstone of GORD management:
Eat smaller, more frequent meals rather than large portions, which can exacerbate delayed gastric emptying and increase reflux risk
Avoid eating within 3–4 hours of bedtime to allow adequate time for gastric emptying before lying down
Identify and limit trigger foods such as caffeine, alcohol, chocolate, spicy foods, citrus, and high-fat meals
Elevate the head of the bed by 15–20 cm using blocks or a wedge pillow to use gravity to reduce nocturnal reflux
Maintain an upright posture for at least 2–3 hours after meals
Avoid tight-fitting clothing around the abdomen that may increase intra-gastric pressure
Pharmacological management may be necessary for symptom control. Proton pump inhibitors (PPIs) such as omeprazole or lansoprazole are first-line treatments for GORD and can be used safely alongside Wegovy. These medications significantly reduce gastric acid production, alleviating heartburn and promoting oesophageal healing. NICE Clinical Guideline 184 recommends PPIs as the most effective medical treatment for GORD symptoms, typically for a 4-8 week course with review and possible step-down thereafter. Antacids or alginates (such as Gaviscon) can provide rapid, short-term relief for breakthrough symptoms.
Dose titration considerations are important. If GORD symptoms emerge or worsen during Wegovy dose escalation, temporarily maintaining the current dose for an additional 4 weeks before increasing may allow better tolerance. Some patients may require a slower titration schedule than standard protocols. According to the SmPC, the 1.7 mg weekly dose may be used as a maintenance dose if 2.4 mg is not tolerated. Communication with the prescribing clinician is essential to individualise the treatment approach and optimise both weight loss outcomes and gastrointestinal tolerability.
Whilst mild reflux symptoms can often be managed with lifestyle modifications and over-the-counter treatments, certain warning signs and symptoms require prompt medical evaluation. Patients taking Wegovy should be aware of situations warranting contact with their GP or healthcare provider.
Seek medical advice if you experience:
Severe or persistent heartburn that does not respond to lifestyle changes and over-the-counter antacids within 2 weeks
Difficulty or pain when swallowing (dysphagia or odynophagia), which may indicate oesophageal inflammation or stricture
Unexplained weight loss beyond expected Wegovy effects, or loss of appetite accompanied by reflux symptoms
Persistent nausea and vomiting that prevents adequate nutrition or hydration
Vomiting blood (haematemesis) or passing black, tarry stools (melaena), which may indicate gastrointestinal bleeding – these require same-day urgent assessment
Severe chest pain, particularly if different from typical heartburn – call 999 immediately to exclude cardiac causes
Symptoms of anaemia such as fatigue, pallor, or breathlessness, which might suggest chronic gastrointestinal blood loss
Red flag symptoms such as dysphagia, unexplained weight loss, gastrointestinal bleeding, or persistent vomiting warrant urgent referral for upper gastrointestinal endoscopy to exclude serious pathology including Barrett's oesophagus, peptic ulceration, or malignancy. NICE Guideline NG12 (Suspected cancer: recognition and referral) recommends urgent (within 2 weeks) endoscopy for patients with dysphagia or those aged 55 and over with weight loss and upper abdominal pain, reflux, or dyspepsia.
Patients should also contact their prescriber if GORD symptoms significantly impact quality of life or if they are considering discontinuing Wegovy due to gastrointestinal side effects. Often, adjustments to the treatment regimen, additional supportive medications, or temporary dose modifications can improve tolerability without necessitating complete cessation of therapy. Regular monitoring and open communication enable healthcare professionals to balance the benefits of weight loss against any adverse gastrointestinal effects.
For individuals with pre-existing severe GORD or those who cannot tolerate Wegovy due to reflux symptoms, several alternative evidence-based weight management strategies are available. The optimal approach depends on individual circumstances, comorbidities, and patient preferences.
Other pharmacological options for weight management include:
Orlistat (available on NHS prescription or over-the-counter as Alli): This lipase inhibitor reduces dietary fat absorption by approximately 30%. Whilst it has a different mechanism to GLP-1 agonists and does not delay gastric emptying, gastrointestinal side effects (particularly steatorrhoea) are common. Weight loss is typically more modest than with Wegovy (approximately 3–5% body weight).
Liraglutide 3.0 mg (Saxenda): Another GLP-1 receptor agonist, though with similar gastrointestinal effects to semaglutide, so may not be suitable if GORD is problematic with Wegovy. NICE TA664 recommends liraglutide only within specialist weight management services.
Naltrexone-bupropion combination (Mysimba): Licensed in the UK but not routinely commissioned by the NHS; acts on central appetite regulation pathways.
Behavioural and lifestyle interventions remain fundamental to weight management and are recommended by NICE as first-line treatment for all patients with obesity:
Structured weight management programmes incorporating dietary modification, increased physical activity, and behavioural strategies
Very low-calorie diets (VLCDs) or low-calorie diets under medical supervision, which can achieve significant short-term weight loss
Psychological support including cognitive behavioural therapy (CBT) to address eating behaviours and emotional factors
Increased physical activity: Aim for at least 150 minutes of moderate-intensity activity weekly, building up gradually, in line with UK Chief Medical Officers' Physical Activity Guidelines
Bariatric surgery represents the most effective intervention for substantial, sustained weight loss in people with severe obesity. NICE Clinical Guideline 189 recommends considering bariatric surgery for people with BMI ≥40 kg/m² or ≥35 kg/m² with comorbidities, with lower thresholds (BMI 30-34.9 kg/m²) for those with recent-onset type 2 diabetes and adjusted thresholds for certain ethnic groups. Interestingly, whilst some bariatric procedures (particularly sleeve gastrectomy) may initially worsen GORD, gastric bypass often improves reflux symptoms. Comprehensive pre-operative assessment includes evaluation of GORD severity.
Patients with GORD seeking weight management should discuss their individual risk-benefit profile with their healthcare provider. In many cases, the metabolic and cardiovascular benefits of weight loss outweigh the temporary inconvenience of managing reflux symptoms, but personalised decision-making is essential.
Wegovy may temporarily worsen GORD symptoms in some patients due to delayed gastric emptying, though weight loss often improves reflux over time. Individual responses vary, and symptoms can usually be managed with lifestyle modifications and proton pump inhibitors.
There is no official contraindication to using Wegovy in patients with GORD. Careful assessment and monitoring are advisable, and many patients find reflux symptoms improve as weight loss progresses.
Start with lifestyle modifications such as eating smaller meals, avoiding food 3–4 hours before bedtime, and elevating the head of your bed. If symptoms persist beyond 2 weeks, contact your GP who may recommend proton pump inhibitors or adjust your Wegovy dose titration.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.