
Deciding when to start GLP-1 medications after surgery requires careful consideration of individual recovery, surgical type, and potential complications. GLP-1 receptor agonists such as semaglutide, liraglutide, and tirzepatide are widely prescribed in the UK for type 2 diabetes and weight management, but their effects on gastric emptying and appetite have important implications for post-operative care. UK guidance emphasises individualised risk assessment and shared decision-making when planning perioperative medication management. This article explores current clinical recommendations, surgical considerations, and safety factors to help patients and healthcare professionals determine the optimal timing for resuming GLP-1 therapy following surgical procedures.
Quick Answer: GLP-1 medications can typically be restarted after surgery once the patient has recovered from anaesthesia, normal gastrointestinal function has returned, and they can tolerate regular oral intake without significant nausea or vomiting.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereGlucagon-like peptide-1 (GLP-1) receptor agonists are a class of medications primarily used to manage type 2 diabetes mellitus and, more recently, for weight management in adults with obesity or overweight with weight-related comorbidities. In the UK, commonly prescribed GLP-1 medications include semaglutide (Ozempic for diabetes, Wegovy for weight management), liraglutide (Victoza for diabetes, Saxenda for weight management), dulaglutide (Trulicity for diabetes), and tirzepatide (Mounjaro for diabetes), which is a dual GLP-1 and GIP receptor agonist.
These medications work by mimicking the action of naturally occurring GLP-1, a hormone released by the intestine in response to food intake. The mechanism of action includes several key effects: they stimulate insulin secretion from pancreatic beta cells in a glucose-dependent manner, suppress glucagon release (which reduces hepatic glucose production), slow gastric emptying, and act on appetite centres in the brain to promote satiety and reduce food intake. This combination of effects leads to improved glycaemic control and, in many patients, significant weight loss.
The gastric emptying effect is particularly relevant when considering the timing of GLP-1 initiation after surgery. By delaying the movement of food from the stomach into the small intestine, GLP-1 medications can affect how the gastrointestinal tract functions during the critical post-operative recovery period. It's worth noting that this gastric emptying delay may attenuate over time with chronic use (tachyphylaxis), though clinically relevant effects often persist. This pharmacological action has important implications for surgical patients, particularly those undergoing procedures requiring general anaesthesia or involving the gastrointestinal system. Understanding these mechanisms is essential for healthcare professionals and patients when planning the safe resumption of GLP-1 therapy following surgical procedures.
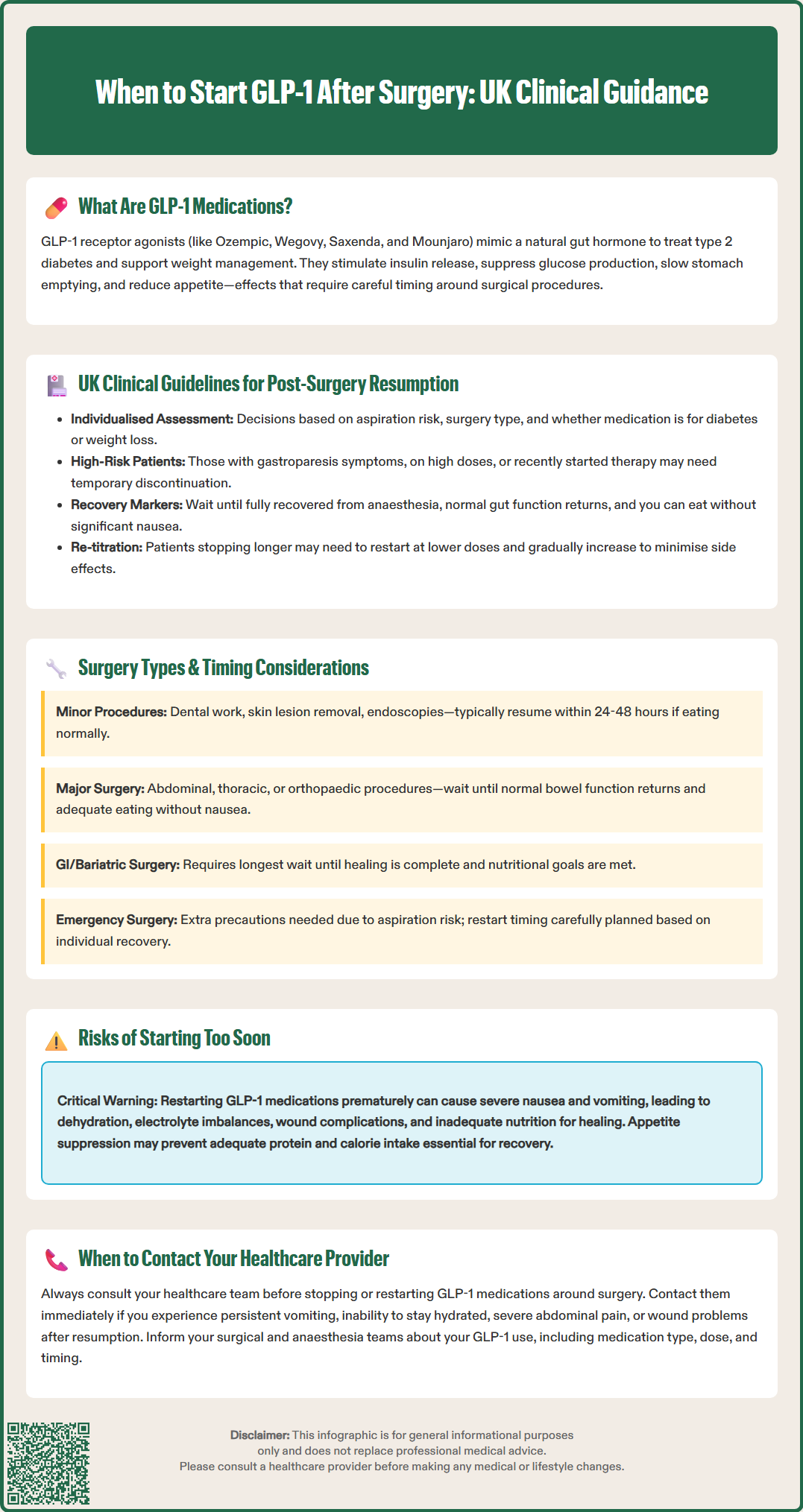
UK guidance on managing GLP-1 medications around surgery is evolving as clinical experience with these agents increases. Current UK practice, as reflected in guidance from the Centre for Perioperative Care (CPOC) and the Joint British Diabetes Societies (JBDS), generally supports continuing GLP-1 receptor agonists perioperatively for most patients, with appropriate risk assessment and precautions.
For patients undergoing surgery, the approach should be individualised based on several factors:
Aspiration risk assessment: Patients with symptoms of gastroparesis, those on high doses, or those who have recently initiated therapy may have increased risk
Type of surgery: Particularly procedures involving the gastrointestinal tract
Indication for GLP-1 therapy: Whether for diabetes management or weight loss
If GLP-1 medications are temporarily withheld based on individual risk assessment or local policy, patients with diabetes should have a clear plan for glycaemic management, including capillary glucose monitoring and potential bridging therapy, developed in consultation with the diabetes team.
Regarding post-operative resumption, there is no single universal timeframe, as the decision depends on multiple factors. Generally, GLP-1 medications can be restarted when:
The patient has fully recovered from anaesthesia
Normal gastrointestinal function has returned
The patient can tolerate regular oral intake without significant nausea or vomiting
Any post-operative complications have resolved
Patients should always consult their healthcare team before restarting these medications. For those who have had a longer interruption in therapy, re-titration from a lower dose may be necessary to minimise gastrointestinal side effects. The NICE guidance on perioperative care (NG180) emphasises the importance of individualised care and shared decision-making throughout the perioperative period.
The type of surgical procedure significantly influences when it is safe to restart GLP-1 medications. Different operations carry varying levels of risk and require different recovery periods before these medications can be safely reintroduced.
Gastrointestinal and bariatric surgery requires particular caution. Following procedures such as gastric bypass, sleeve gastrectomy, or bowel resection, the gastrointestinal tract needs time to heal and adapt. The gastric emptying delay caused by GLP-1 medications could potentially interfere with surgical healing, increase nausea and vomiting, or compromise nutritional intake during the critical early recovery phase. For these procedures, the decision to restart GLP-1 therapy should be guided by the surgical team and dietitian, typically when the patient has advanced to an appropriate diet stage and is meeting nutritional goals. The British Obesity and Metabolic Surgery Society (BOMSS) guidance may provide specific recommendations for medication management after bariatric procedures.
Major abdominal, thoracic, or orthopaedic surgery generally requires waiting until the patient has returned to normal bowel function and can maintain adequate oral nutrition without significant nausea before reintroducing medications that may suppress appetite or delay gastric emptying. The timing should be individualised based on the patient's recovery rather than following a fixed timeline.
Minor surgical procedures performed under local anaesthesia or brief general anaesthesia (such as dental surgery, skin lesion removal, or endoscopic procedures) often allow continuation or early resumption of GLP-1 medications, potentially within 24-48 hours, provided the patient has recovered well and can eat and drink normally.
Emergency surgery presents unique challenges, as patients may have recently taken their GLP-1 medication. In these cases, anaesthetists take additional precautions to reduce aspiration risk, and post-operative resumption is carefully planned based on individual recovery. Patients should always inform their surgical and anaesthetic teams about their GLP-1 medication use, including the type, dose, and when they last took it.
Restarting GLP-1 medications prematurely after surgery can lead to several significant complications that may compromise recovery and patient safety. Understanding these risks is essential for both healthcare professionals and patients.
Increased nausea and vomiting represents one of the most common complications. GLP-1 medications inherently cause nausea in many patients, particularly during initiation or dose escalation. In the post-operative period, when patients may already experience surgical-related nausea, adding a GLP-1 agonist can exacerbate symptoms significantly. Persistent vomiting can lead to dehydration, electrolyte imbalances, delayed wound healing, and increased risk of surgical site complications such as wound dehiscence (separation of wound edges).
Delayed gastric emptying poses particular concerns in the post-operative setting. The slowed movement of stomach contents can interfere with nutritional intake at a time when adequate nutrition is crucial for healing. This effect may also increase the risk of aspiration if a patient requires additional procedures or experiences complications requiring urgent intervention. Furthermore, symptoms of delayed gastric emptying may overlap with post-operative complications such as bowel obstruction, potentially complicating diagnosis and requiring careful clinical assessment.
Compromised nutritional status and hydration can occur when GLP-1-induced appetite suppression and early satiety prevent patients from meeting their post-operative nutritional requirements. Adequate protein and calorie intake is essential for wound healing, immune function, and recovery of strength. Reduced fluid intake due to nausea and early satiety can contribute to dehydration risk.
Medication interactions and absorption issues may arise with some GLP-1 medications. While most GLP-1 receptor agonists have minimal clinically relevant effects on the absorption of other medications, tirzepatide can reduce exposure to some oral medicines, including oral contraceptives, as noted in its Summary of Product Characteristics.
Patients should contact their healthcare provider immediately if they experience persistent vomiting, inability to maintain hydration, severe or persistent abdominal pain (which could indicate pancreatitis, a rare but serious side effect), bilious vomiting, signs of wound complications, or inability to keep fluids down. Suspected adverse effects should be reported via the MHRA Yellow Card Scheme. A cautious, individualised approach to resumption, guided by clinical assessment, minimises these risks and supports optimal surgical recovery.
The timing depends on your individual recovery, surgery type, and ability to tolerate normal oral intake. For minor procedures, resumption may be possible within 24-48 hours, whilst major or gastrointestinal surgery may require longer. Always consult your healthcare team before restarting.
Current UK guidance generally supports continuing GLP-1 medications perioperatively for most patients, with appropriate risk assessment. Your surgical and anaesthetic teams will advise based on your individual circumstances, surgery type, and aspiration risk.
Starting GLP-1 medications prematurely can cause increased nausea and vomiting, delayed gastric emptying, compromised nutritional intake, dehydration, and potentially delayed wound healing. These effects may interfere with post-operative recovery and increase complication risk.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.