
Glucagon-like peptide-1 (GLP-1) receptor agonists, including semaglutide (Ozempic, Wegovy), dulaglutide (Trulicity), and liraglutide (Victoza, Saxenda), are injectable biological medicines used for type 2 diabetes and weight management. A frequent question is: do GLP-1 have to be refrigerated? Yes, most GLP-1 medications require refrigeration at 2–8°C before first use to preserve their protein-based structure and therapeutic effectiveness. Storage requirements differ between single-use and multi-dose pens, and some products permit limited room temperature storage. Understanding proper storage—before opening, after first use, and during travel—ensures your medication remains potent, safe, and clinically effective throughout its shelf life.
Quick Answer: Most GLP-1 receptor agonists must be refrigerated at 2–8°C before first use, though some permit limited room temperature storage, and multi-dose pens often allow room temperature storage after opening.

Mounjaro® is the most innovative GLP-1 medication proven to dramatically curb appetite, hunger, and cravings to help professional men achieve substantial weight loss.
Start Here
Wegovy® is a weekly injectable GLP-1 medication with proven effectiveness in reducing appetite, hunger, and cravings to help busy professionals lose significant weight.
Start HereGlucagon-like peptide-1 (GLP-1) receptor agonists are a class of injectable medications used primarily for managing type 2 diabetes and, in some formulations, for weight management. These biological medicines include semaglutide (Ozempic, Wegovy), dulaglutide (Trulicity), liraglutide (Victoza, Saxenda), and exenatide (Byetta, Bydureon). A common question among patients prescribed these medications is whether refrigeration is necessary.
The short answer is yes, most GLP-1 medications require refrigeration before first use. These are protein-based biological products that can degrade when exposed to temperatures outside their recommended storage range. Maintaining proper storage conditions is essential to preserve the medication's potency, safety, and effectiveness throughout its shelf life.
It's important to understand that GLP-1 medications come in two main types: single-use pens (like Wegovy, Trulicity, and Bydureon BCise) that are used once and discarded, and multi-dose pens (like Ozempic, Victoza, Saxenda, and Byetta) that are used multiple times until empty. This distinction affects storage requirements.
While refrigeration is generally required for unopened pens, some GLP-1 medications can be kept at room temperature for limited periods before first use, and multi-dose pens often have different storage requirements once opened and in use. The Medicines and Healthcare products Regulatory Agency (MHRA) and manufacturers provide detailed storage instructions in the patient information leaflet supplied with each medication. Following these guidelines precisely ensures the medication remains stable and effective.
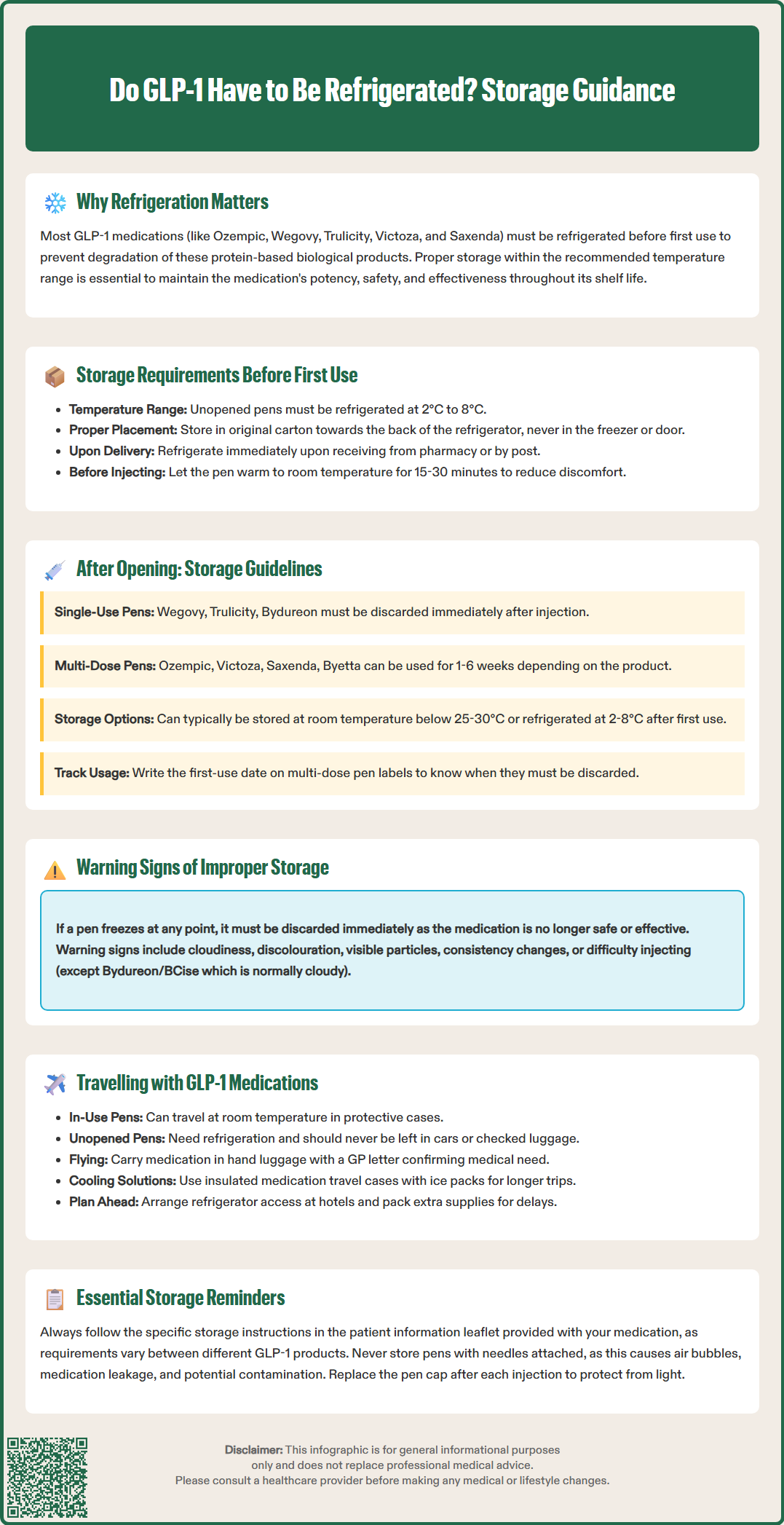
Unopened GLP-1 medication pens must generally be stored in a refrigerator at temperatures between 2°C and 8°C until you are ready to use them for the first time. This temperature range is critical for maintaining the structural integrity of the peptide molecules within the medication.
However, several GLP-1 medications can be kept at room temperature for limited periods before first use, according to their specific product information:
Wegovy (semaglutide): Unopened pens may be kept at temperatures up to 30°C for up to 28 days before use
Trulicity (dulaglutide): Unopened pens may be kept at temperatures up to 30°C for up to 14 days before use
Ozempic, Victoza, Saxenda: Check the specific product information for allowable room temperature storage before first use
When storing unopened pens in the refrigerator:
Keep the pen in its original carton to protect it from light exposure, which can degrade the active ingredient
Store towards the back of the refrigerator rather than in the door, where temperature fluctuations are more common
Never place the pen in the freezer compartment or allow it to freeze; frozen GLP-1 medications must be discarded
Keep away from the freezer element to prevent accidental freezing
Check the expiry date printed on the carton and pen label before use
If you receive your GLP-1 medication by post or collect it from a pharmacy, refrigerate it as soon as possible. If you're concerned about delivery delays or if products arrive warm, contact your pharmacy for advice.
Before your first injection, you may wish to remove the pen from the refrigerator and allow it to reach room temperature for approximately 15–30 minutes. Injecting cold medication can cause discomfort at the injection site, though this does not affect the medication's efficacy. Always inspect the solution before use—most GLP-1 medications should be clear and colourless (Bydureon/BCise is an exception, becoming a cloudy suspension after mixing). Do not use if the solution appears inappropriately cloudy, discoloured, or contains particles.
Storage requirements after first use vary significantly between GLP-1 products, particularly between single-use and multi-dose pens:
Single-use pens (used once and then discarded):
Wegovy (semaglutide): Single-use only. After injection, discard the pen. Unopened pens may be kept at temperatures up to 30°C for up to 28 days before use.
Trulicity (dulaglutide): Single-use only. After injection, discard the pen. Unopened pens may be kept at temperatures up to 30°C for up to 14 days before use.
Bydureon/Bydureon BCise (exenatide prolonged-release): Single-use only. After reconstitution, use immediately and then discard the pen.
Multi-dose pens (used multiple times until empty):
Ozempic (semaglutide): May be stored at room temperature (below 30°C) or in the refrigerator (2–8°C) for up to 6 weeks after first use.
Victoza, Saxenda (liraglutide): May be stored at room temperature (below 30°C) or refrigerated for up to 1 month after opening.
Byetta (exenatide): Should be stored below 25°C after first use and can be used for 30 days. Follow the patient information leaflet regarding whether to return to refrigeration.
Important storage practices for all pens:
Always replace the pen cap after each injection to protect from light
Store away from direct heat sources such as radiators, windowsills, or car glove compartments
Keep out of direct sunlight, which can degrade the medication
Do not store with the needle attached, as this can cause air bubbles, leakage, or contamination
Write the date of first use on multi-dose pen labels to track the discard date
For multi-dose pens, follow the specific product information regarding whether refrigeration or room temperature storage is preferred after opening. Some products have specific recommendations about temperature consistency during the in-use period.
Improper storage of GLP-1 medications can compromise their therapeutic effectiveness and potentially lead to inadequate glycaemic control or reduced weight management efficacy. Understanding the consequences of storage errors helps patients recognise when medication replacement may be necessary.
If a GLP-1 pen has been frozen, the medication must be discarded immediately, even if it subsequently thaws. Freezing causes irreversible changes to the protein structure of GLP-1 molecules, rendering the medication ineffective. The product information for all GLP-1 medications clearly states that frozen products should not be used.
Exposure to excessive heat (temperatures above the recommended storage limits) can also degrade the active ingredient. If an unopened pen has been kept at room temperature beyond the specific time allowed in its product information, or if an in-use multi-dose pen has exceeded its room temperature storage period, contact your pharmacist or diabetes specialist nurse for advice. They can assess whether the medication remains usable or requires replacement.
Signs that a GLP-1 medication may have been compromised include:
Cloudiness or discolouration of the solution (except for Bydureon/BCise, which is normally cloudy after reconstitution)
Visible particles or crystals in the liquid
Changes in solution consistency
Difficulty injecting or unusual resistance
If you suspect your medication has been stored incorrectly, do not use it without professional guidance. Using degraded medication may result in:
Suboptimal blood glucose control in patients with type 2 diabetes
Reduced weight loss efficacy for those using GLP-1 agonists for weight management
Unpredictable dosing, as degradation may be partial rather than complete
Patients with diabetes who suspect reduced medication efficacy should monitor their blood glucose more frequently and contact their GP or diabetes care team if they have concerns about medication storage or if their diabetes control deteriorates unexpectedly.
Travelling with GLP-1 medications requires advance planning to maintain proper storage conditions whilst away from home. Whether travelling domestically within the UK or internationally, patients can successfully manage their medication with appropriate preparation.
For short trips (day trips or overnight stays):
In-use multi-dose pens can be carried at room temperature in their protective case, within their permitted in-use period
Unopened pens should generally be kept refrigerated, though some may be kept at room temperature for limited periods (check your specific product information)
Use an insulated medication travel case to protect from temperature extremes
Avoid leaving medication in cars, particularly during warm weather or in direct sunlight
Keep medication in hand luggage when flying, never in checked baggage where temperatures may fall below freezing
For longer journeys requiring refrigeration:
Use a medical-grade cooling case or insulated travel wallet with ice packs designed for medication transport
Avoid direct contact between ice packs and medication pens, as this may cause freezing; use a barrier layer
Request refrigerator access at hotels or accommodation in advance
Consider portable medication coolers that maintain 2–8°C for extended periods
When flying with GLP-1 medications:
Carry a letter from your GP or prescriber confirming your need for injectable medication and refrigeration
Keep medication in original packaging with pharmacy labels clearly visible
Inform security staff that you are carrying medical injections
Pack extra supplies including needles and alcohol swabs in case of travel delays or lost luggage
Research refrigeration options at your destination before departure
Plan for safe sharps disposal during your trip
The NHS advises patients to carry sufficient medication for their trip plus additional supplies in case of unexpected delays. If travelling to countries with extreme temperatures, consult your pharmacist about specialised cooling solutions. For extended travel where refrigeration is unavailable, discuss timing your trip around your injection schedule with your healthcare provider, though this should not compromise your treatment regimen.
For more information, visit the NHS pages on 'Taking medicines abroad' and 'How to store medicines'.
No, GLP-1 medications that have been frozen must be discarded immediately, even after thawing. Freezing causes irreversible damage to the protein structure, rendering the medication ineffective and unsafe to use.
This varies by product: Wegovy may be kept at up to 30°C for 28 days before use, whilst Trulicity permits up to 14 days at room temperature. Always check your specific product information leaflet for precise guidance.
Storage after opening depends on the product. Multi-dose pens like Ozempic and Victoza may be stored at room temperature (below 30°C) or refrigerated for up to 6 weeks and 1 month respectively. Single-use pens like Wegovy and Trulicity are discarded immediately after injection.
All medical content on this blog is created based on reputable, evidence-based sources and reviewed regularly for accuracy and relevance. While we strive to keep content up to date with the latest research and clinical guidelines, it is intended for general informational purposes only.
DisclaimerThis content is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional with any medical questions or concerns. Use of the information is at your own risk, and we are not responsible for any consequences resulting from its use.